Cover Story | Structural Heart Intervention: A Peek at the Future
Building on the success of transcatheter aortic valve replacement (TAVR), complex percutaneous structural heart interventions are increasingly providing options to patients who previously had limited options and, in some patients, replacing open surgery for the treatment for structural heart diseases (SHD).
Three visionary interventionalists who are leading the way in innovation and transformation in this field highlight some of the key trends to watch in structural heart interventions over the next decade.
Tricuspid Regurgitation: The Next Frontier
Tricuspid regurgitation (TR) has historically been an overlooked area in valvular heart disease. However, in recent years, it has become evident that TR is more prevalent than previously believed and carries significant morbidity and mortality risks. With advancements in imaging technology, a deeper understanding of tricuspid valve anatomy, and the development of transcatheter therapies for TR treatment, the tricuspid valve is an emerging key area of focus in the catheterization laboratory.
"Much of our work until now was around creating percutaneous equivalents to surgery, which is a valid goal. But over the next 10 years or so, we have some interesting opportunities to explore areas where there is no surgical predicate. The tricuspid valve is the most important of these areas," says James M. McCabe, MD, FACC, from the University of Washington.
Looking at Medicare billing data, the average cardiothoracic surgery program in the U.S. does only five or six isolated tricuspid valve surgeries per year, "which is to say they do none," he says.
"In the tricuspid space, outside of congenital heart disease and bacterial endocarditis, everything is brand new. There is much we don't know about the tricuspid valve and the interplay between the tricuspid and the right ventricle, so we're going to define objectives, metrics and parameters about how to evaluate outcomes as we go," McCabe adds.
Besides the general sense that the tricuspid valve may not affect symptoms or long-term outcomes, the data from isolated repair are modestly encouraging. Reported mortality in the surgical literature has traditionally hovered around 10%, although mortality as low as 3.2% has been reported in a contemporary surgical series.1
"Looking at the incidence of the disease state, moderate or greater TR is the most common valvular heart problem, followed by mitral regurgitation (MR), followed by aortic stenosis. So there is definitely an unmet need," notes McCabe. "But before we can start treating all these people, we need to see a clear demonstration of the value proposition of tricuspid intervention."
The TriClip device (Abbott Vascular, Santa Clara, CA) offers direct restoration of leaflet coaptation and should be approved soon for tricuspid repair. In McCabe's view, the device's future took a meaningful but not massive step forward with the pivotal TRILUMINATE trial, presented at ACC.23/WCC and simultaneously published in the New England Journal of Medicine.2

– Jame M. McCabe, MD, FACC
In TRILUMINATE, 350 patients with symptomatic severe TR were randomly assigned to receive either tricuspid transcatheter edge-to-edge repair (T-TEER) or medical therapy. About 70% of patients had either 'massive' or 'torrential' TR.
For a hierarchical primary endpoint that included all-cause death, tricuspid valve surgery, hospitalization for heart failure (HF) and quality of life, T-TEER was favored over medical therapy, with the benefit driven by improvement in quality of life and no significant differences seen in the clinical endpoints.
Importantly, T-TEER was found to be safe; 98.3% of the patients who underwent the procedure were free from major adverse events at 30 days. At 30 days, 87.0% of the patients in the TEER group and 4.8% of those in the control group had TR of ≤ moderate severity (p<0.001).
"How I read that paper was that it's early days and most operators in the trial were placing tricuspid clips for the first time and it's challenging to do," notes McCabe.
"As we might expect, people who had good outcomes felt much better. Perhaps we'll see those are the ones who can stay out of the hospital and survive longer. Unfortunately, in the study there were only 175 treated patients, so when slicing and dicing into the degree of improvement in TR, the numbers get very small. I don't think this trial told us everything we need to know about treating tricuspid valves," says McCabe.
Besides the TriClip, another leaflet coaptation device, the PASCAL transcatheter valve repair system (Edwards Lifesciences, Irvine, CA) has been successfully used to reduce TR, according to Samir R. Kapadia, MBBS, FACC, from the Cleveland Clinic Foundation. There are also a few valve replacement devices that are performing "fairly" well in early trials.
Imaging: What You Get is What You See
In early days, imaging in structural heart intervention was largely limited to intracardiac transesophageal echocardiography (TEE). This is no longer the case.
"In cardiac surgery, you need proper exposure of the anatomy to do your repair or replacement," says Kapadia. "The concept is the same in structural intervention, except the exposure comes through procedural imaging. You need to see the field with 3D accuracy."
Imaging has played an important role in the clinical evolution of TAVR, specifically with the use of multidetector computed tomography (MDCT) to help with transcatheter valve sizing, as well as for patient selection and the identification of early signs of prosthesis pathology.3
Advanced imaging is also driving progress in the mitral space, with 4D TEE guiding pre-planning and procedural placement of edge-to-edge repair devices for MR.

– Samir R. Kapadia, MBBS, FACC
For repair of TR, imaging has an even more important role given the complexity of the anatomy and the unique challenges of right-sided valve intervention. As a crude measure of the complexity involved in imaging for tricuspid repair and replacement, a recent review on the topic by Agricola, et al., stretched more than 50 pages.4
"As we progress from simple procedures to more complex procedures, the imaging needs are much greater. The aortic valve is relatively simple, so the imaging needed to intervene is also relatively simple. But when thinking about mitral repair and replacement, right-sided tricuspid intervention, or looking at making incisions into leaflets and implanting devices within the left ventricle (LV) for HF, it's easy to imagine that the imaging needs are much greater," says McCabe.
Intracardiac echocardiography (ICE) is a newer imaging modality, costlier than TEE but providing operators with high-resolution real-time visualization of cardiac structures, continuous monitoring of catheter location within the heart, shorter procedure time, and reduced fluoroscopy time. ICE can also alert operators to procedural complications, like pericardial effusion or thrombus formation.
ICE has provided an important option to replace or complement TEE as the preferred imaging modality for guiding certain procedures. More recently, 3D and 4D volumetric ICE systems show particular promise in structural interventions.
One issue McCabe sees as imaging gets progressively more complex is the need for more advanced imaging expertise both before and during procedures.
"I think we need to think more in terms of partnerships and a multidisciplinary approach to SHD imaging, where we evolve from having just one interventional cardiologist to an interventional cardiologist and an invasive imager, working together. It's just getting to be too much for one person to manage."
There seems to be some consensus around an impending labor shortage in this area. "As structural heart procedures expand to new sites around the country, it has become clear that there are not enough structural heart imaging experts who are fully trained in both MDCT and TEE techniques for the evaluation and procedural guidance of these increasingly complex patients, procedures, and devices," write Paul A. Grayburn, MD, FACC, and Y.S. Chandrashekhar, MD, DM, FACC, in a recent JACC: Cardiovascular Imaging review on the topic.3
(Noninvasive) Lifetime Management of SHD
Despite the high prevalence of valvular heart disease and its clear impact on survival and quality of life, there is very little awareness of it among the general population. Also, the easiest and most cost-effective way to diagnose valvular heart disease remains cardiac auscultation, a technique no longer routinely performed at the primary care provider level and, obviously, not a diagnostic that translates well to a telemedicine model.
Added to this, there is clear evidence now that late referral, conservative management and gross undertreatment of valvular disorders, while perhaps once appropriate given the risks involved with surgery, is no longer appropriate for most patients.5
"Things are very different today than even from a decade ago. Minimally invasive approaches are becoming a reality where we're using methods to reach the heart via blood vessels and correct anatomical problems. For patients and physicians to embrace these technologies, they do not have to be better than conventional surgery, they only have to be as safe and noninferior in efficacy," says Kapadia. All of the PARTNER TAVR trials, for example, were noninferiority trials.
This noninvasiveness also allows structural heart interventionalists to adopt a stepped or sequential approach to treatment, marking a further move away from the surgical experience.

– Adam B. Greenbaum, MD, FACC
"In surgery you need to do everything possible all at once. If you have a patient with moderate aortic stenosis and you are doing coronary bypass surgery, there's a class I indication to replace the aortic valve at the same time," says Kapadia.
On the transcatheter side, there is greater flexibility. "For a patient with MR, you might opt to try indirect annuloplasty with a CARILLON device, which is relatively easy to implant and low risk, and see if the regurgitation improves. If that doesn't work then you can do a MitraClip and perhaps go on to a mitral valve replacement, if needed, down the line."
The lifetime management of SHD represents an important paradigm shift in the field, particularly as TAVRs and mitral repairs and replacements are increasingly being done on lower risk, younger, less sick patients. Now, says Kapadia, the focus is on managing a patient's valvular heart disease for 25 years or more rather than thinking in terms of a one-time intervention.
Abandoning the one-and-done approach can also facilitate more appropriately-timed intervention, adds McCabe. "When we were more dependent on surgery to fix things, having some things done earlier than strictly necessary was sort of the cost of doing business. If you had to violate the chest, you were going to get everything done, even if you were a couple years early for, say, replacing the mitral valve concurrent with an aortic valve replacement. Being able to stage things really moves us closer to that ideal of precision therapy."
Transcatheter Surgery
For Adam B. Greenbaum, MD, FACC (Emory University), the future of structural heart disease treatment looks less like the operating room on Grey's Anatomy and more like the Sickbay on the Starship Enterprise.
"Where I see us moving is from our current job, which is basically limited to deployment of devices and anchors, to doing actual surgical procedures but through blood vessels," he says.
"What is it that surgeons do? They open the chest and cut things out, they put in stiches and sew, they use pledgets. What we're trying to do now is figure out how to mimic these techniques percutaneously."
Greenbaum is referring to transcatheter electrosurgery, which uses alternating current in the radiofrequency range (about 500 kHz) to heat or vaporize tissue and therefore traverseor lacerate tissue. (Not to be confused with catheter ablation, which uses radiofrequency energy to form nonconductive lesions but not to vaporize tissue.)
Importantly, electrosurgery techniques limit collateral tissue damage and the short duration of energy used prevents tissue desiccation and coagulum formation.
"My group is using specialized catheters to perform similar maneuvers and procedures to what surgeons do. So, for example, we started lacerating leaflets because there were people who weren't candidates for either a transcatheter aortic valve replacement or transcatheter mitral valve replacement because the leaflet was in the way. So we started using electricity to cut the leaflets to get them out of the way, making that patient now a candidate for valve intervention," says Greenbaum.
BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent artery obstruction) uses radiofrequency energy to lacerate the aortic valve leaflets to prevent occlusion of the coronary ostium during native or valve-in-valve TAVR. LAMPOON (laceration of the anterior mitral leaflet to prevent outflow obstruction) involves the laceration of the anterior mitral leaflet to prevent LV outflow obstruction after mitral valve replacement.
"Our success with procedures like BASILICA and LAMPOON led us to ask what else we could cut. Recently we've actually started cutting muscle and we've developed a procedure called SESAME, which mimics surgical myectomy performed to treat hypertrophic cardiomyopathy," he adds.
Rather than resecting muscle in hypertrophied hearts, SESAME (septal scoring along the midline endocardium) uses wires to navigate inside the myocardium and perform a transcatheter myotomy that mimics the muscle resection done in the operating room. "We're seeing improvements in symptoms with these patients without having to perform open surgery," says Greenbaum, who published a first-in-human report last summer.6 SESAME is also being proposed as a means to avoid LV outflow tract obstruction after transcatheter mitral valve replacement.
"Electrosurgery really represents a versatile family of procedures and there are all sorts of things going on. For example, there are case reports of using electrified snares to remove tumors from inside the heart. A snare is advanced around the neck of the tumor, the snare is electrified and then secured around the tumor to pull it out. So now instead of what would be an open surgery for a tumor removal, it can be done through the blood vessels."
Mending a Broken Heart Without Surgery
One dream, says Greenbaum, is to be able to provide transcatheter solutions for HF, both systolic and diastolic. "HF is the end result of all coronary disease, all hypertension, all valvular heart disease, all congenital heart disease. Now we're asking what unique strategies can we bring to HF, not just medically, but mechanically?"
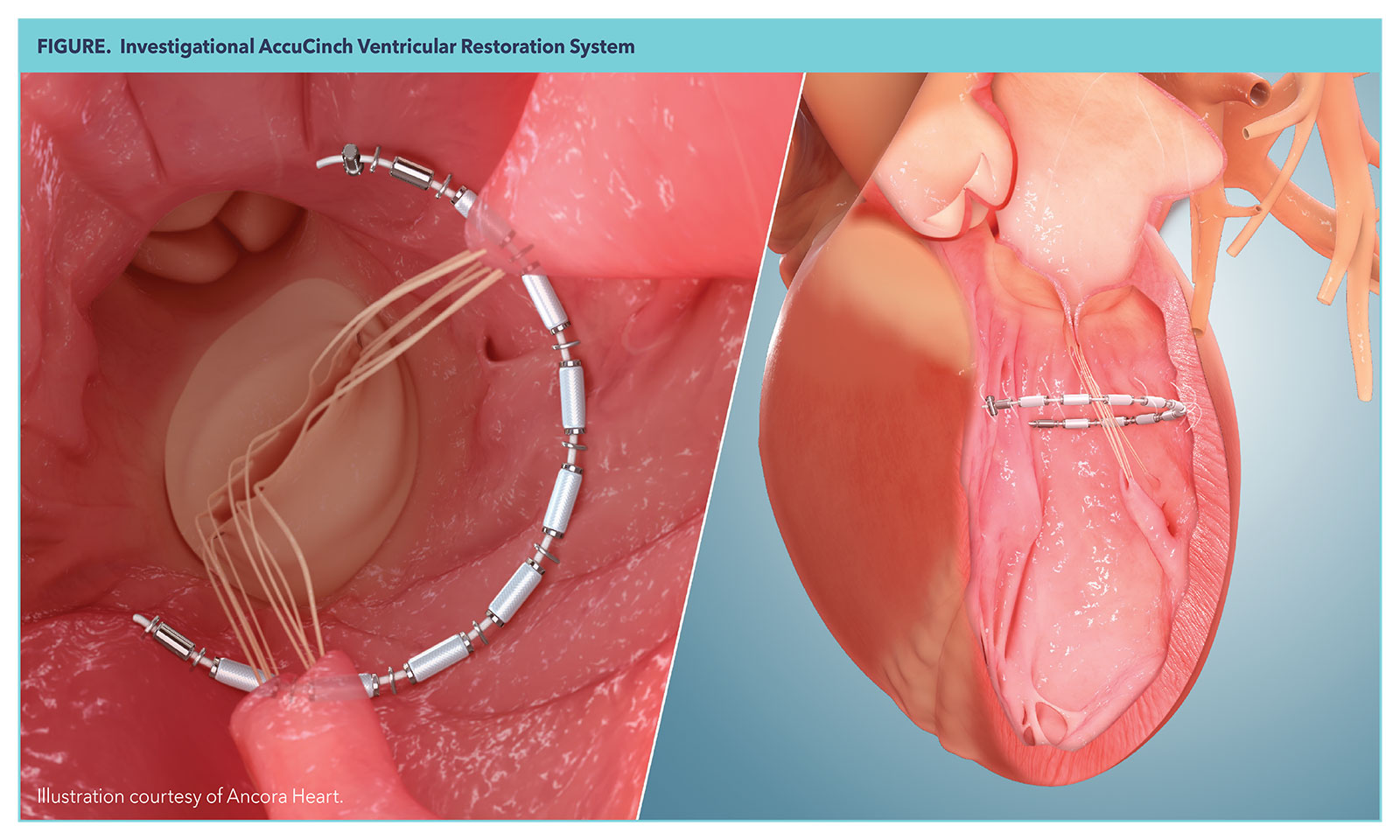
One device now in pivotal trials is the AccuCinch Ventricular Restoration System (Ancora Heart, Santa Clara, CA), which is being proposed as a treatment option filling the gap between medical therapy or cardiac resynchronization therapy and left ventricular assist device implantation or heart transplantation. AccuCinch has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration.
The AccuCinch is basically a posterior brace for the LV. It is delivered to the LV and anchored down by a series of anchors, each separated by a spacer to distribute force, delivered over a cable and placed into the LV wall. The implant is then cinched and secured with a nitinol lock to reduce the size of the LV and reduce wall stress, with the hope of initiating reverse remodeling to improve myocardial contractility.
The early feasibility data on this device are promising, showing it may improve quality of life, functional capacity, and enable reverse remodeling in patients with HF and reduced ejection fraction. The CORCINCH-HF trial, which is currently enrolling, will compare the AccuCinch plus guideline-directed medical therapy (GDMT) to GDMT alone in 400 patients from up to 80 centers worldwide.
There are other novel devices in development that also alter LV geometry or create anatomic shunts to decompress the left atrium.
Clean Up on Aisle 5
With the move towards lifetime management, and addressing issues sequentially, earlier in the disease process and in younger people, removing unwanted devices is a growing issue.
"You can't just keep stacking things in the body. It gets crowded and the areas smaller, so we need more focus on ex-plantation of devices that are no longer working well or no longer needed," notes McCabe. "We are increasingly good at putting things in the body, but we have not sorted out how to get stuff out."
This is an area ripe for innovation, he says, but also one where electrosurgery as a surgical imitation might be useful. "In surgery you cut out what you don't want before something new goes in. Those of us working with transcatheter electrosurgery are working on doing that percutaneously."
Continual Innovation in Valve Technology
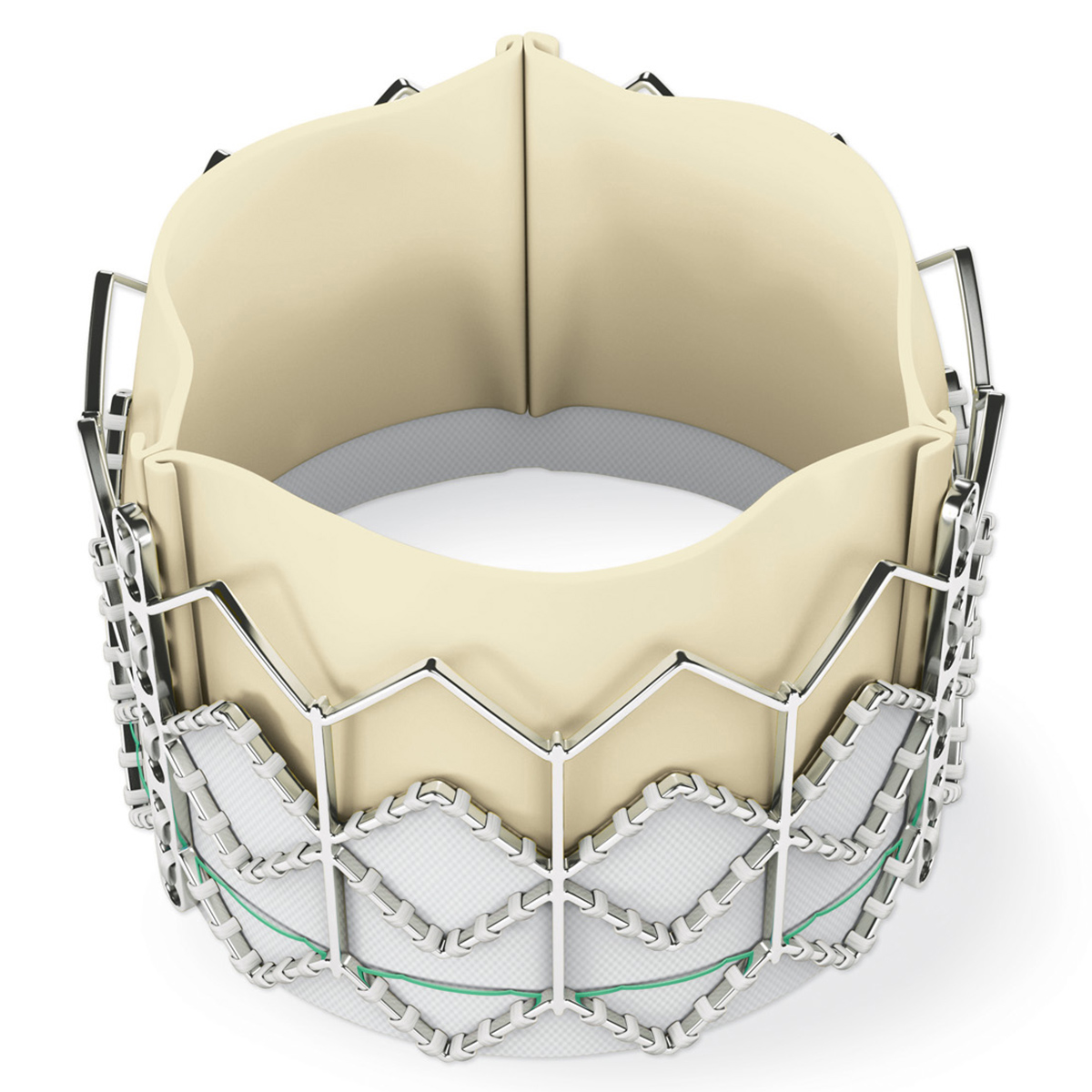
One thing the field of structural intervention will never lack for is valve innovation. This is because it is an extremely profitable business for device makers.
"Innovation and precision in devices for structural heart intervention has not been a challenge for the simple reason that it's such a good business for the device makers," says Kapadia. "In our cath lab, for example, we do close to 650 or 700 TAVRs a year, which at $30,000 per device generates about $20 million of business for device makers. Although we are one of the highest volume centers, this is still a very large number."
In comparison, the amount spent on coronary stents at the Cleveland Clinic is approximately $2 million per year.
The substantial profits generated from valve innovation also have a trickle-down effect on imaging. As valve procedures become more common and more complex, more flexible and precise imaging is needed, resulting in the development of new technologies and technique.
"This is not to say we don't have unmet needs in percutaneous valves. We do, but I'm confident we'll continue to see innovation in this area and get those needs met," says Kapadia.
A New Era in Heart Intervention
It is abundantly clear that structural heart intervention has evolved significantly from its initial goal of mimicking surgical procedures noninvasively. Instead, interventionalists are now reimagining surgery as a noninvasive procedure (transcatheter electrosurgery), boldly going where surgeons have hesitated (the tricuspid valve), and finding new approaches to mechanically supporting the LV, an area where surgery has thus far failed to make meaningful progress. At the same time, there is a growing potential for earlier and more timely interventions to halt disease processes before irreversible heart damage occurs.
This article was authored by Debra L. Beck, MSc.
References
- Hamandi M, Smith RL, Ryan WH, et al. Outcomes of Isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg 2019;108(1):11-15.
- Sorajja P, Whisenant B, Hamid N, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med 2023;March 4. doi:10.1056/NEJMoa2300525.
- Grayburn PA, Chandrashekhar YS. Continuing advances and challenges of structural heart imaging. JACC Cardiovasc Imag;14(:128-30.
- Agricola E, Asmarats L, Maisano F, et al. Imaging for tricuspid valve repair and replacement. JACC Cardiovasc Imag 2021;14:61-111.
- Messika-Zeitoun D, Baumgartner H, Burwash IG, et al. Unmet needs in valvular heart disease. Eur Heart J 2023;March 16. Doi:10.1093/eurheartj/ehad121.
- Greenbaum AB, Khan JM, Bruce CG, et al. Transcatheter myotomy to treat hypertrophic cardiomyopathy and enable transcatheter mitral valve replacement: First-in-human report of septal scoring along the midline endocardium. Circ Cardiovasc Interv 2022;15:e012106.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Pericardial Disease, Valvular Heart Disease, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Cardiac Surgery and VHD, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Interventions, Interventions and Imaging, Interventions and Structural Heart Disease, Computed Tomography, Nuclear Imaging, Mitral Regurgitation
Keywords: ACC Publications, Cardiology Magazine, Tricuspid Valve, Tricuspid Valve Insufficiency, Mitral Valve, Mitral Valve Insufficiency, Transcatheter Aortic Valve Replacement, Quality of Life, Incidence, Medicare, Heart Valve Diseases, Aortic Valve Stenosis, Heart Defects, Congenital, Catheterization, Catheter Ablation, Hospitalization, Endocarditis, Surgical Instruments, Cardiologists, Aortic Valve, Thrombosis, Catheters, Fluoroscopy, Prostheses and Implants, Tomography Scanners, X-Ray Computed, Tomography, X-Ray Computed, Pericardial Effusion, Endocardium, Electrosurgery, Myocardium, Coronary Disease, Ventricular Dysfunction, Left, Stents
< Back to Listings


