Cover Story | Trends in Advanced Heart Failure Therapy: Update on Cardiac Transplantation
Advanced heart failure (AHF) and transplant cardiology is an exciting field these days. Advances are seen across the board – in medical therapy for AHF, donor selection, organ retrieval and preservation, mechanical circulatory support, organ allocation, and more.
Cardiology spoke with three leaders in the field to understand how the treatment of AHF is changing, what's new in heart transplantation, and the role of the nonspecialist in all of it.
Expanding the Donor Pool: An Igloo and a Pig
Heart transplantation in 2022 is both surprisingly low-tech and high-tech. On one hand, most donor hearts are still transported on ice in an Igloo cooler. On the other hand, just a few months ago, a gene-edited pig heart was successfully transplanted into an adult patient.
A continual struggle remains between the supply of donor hearts and demand, but overall, heart transplants have increased 60% in the U.S. since 2012. Even the headwinds provided by the pandemic didn't slow things; heart transplant numbers have grown between 3% and 4.3% every year since 2019. In 2021, there were 3,817 heart transplants reported to UNOS, up from 3,658 in 2020.
For more on the ethical challenges related to heart transplantation, left ventricular assist devices and mechanical circulatory support, click here for a feature from the June issue of Cardiology.
Sadly, one factor expanding the donor pool is the opioid epidemic. In 2021, deaths due to overdose in the U.S. were up 15% from a previous high in 2020. With this increased availability of hearts from younger donors with few or no comorbidities, however, has come the issue of whether to consider for transplantation hearts from individuals with hepatitis C virus (HCV) infection, as well as hepatitis B virus and HIV, and even COVID-positive donors.
For HCV, the availability of direct-acting antivirals, which offer cure rates in excess of 95% with short courses of well-tolerated oral therapy, has changed everything. "We are increasingly able to transplant organs from HCV-positive donors, even donors who are viremic, because we can now offer oral therapies to heart transplant recipients that are both safe and efficacious," says Ersilia M. DeFilippis, MD, from Columbia University Irving Medical Center in New York.
The long-term effects on survival and post-transplant outcomes are still unknown, she adds, but early outcomes look good, with similar 30-day and one-year mortality.1 Notably, in one study, patients who consented to receive an HCV-positive organ had a median wait time of four days compared with 28 days for those accruing waitlist time before consenting to an HCV-positive organ.
Better Organ Preservation
On a happier note, novel organ preservation technologies are also helping increase the number of hearts available for transplant (Figure 1). Traditional cold storage of donor organs (i.e., a cooler, a plastic bag, some ice and a biohazard label) is associated with reperfusion injury and ischemia with longer travel times.
In a presentation at the 2022 International Society for Heart and Lung Transplantation meeting in May, new data on the SherpaPak cardiac transport system (Paragonix Technologies) showed a one-year survival of 96.4% compared with 88.7% for those who received cold-storage hearts (p=0.03).2 The study included 569 adult heart recipients (from October 2015 to January 2022) and utilized propensity scoring.
"Innovations in patient care are rarely this impactful," said primary investigator Marzia Leacche, MD, surgical director at Spectrum Health in Michigan, in a press release. "We hypothesize that the survival benefit is due to reduction in the incidence of severe primary graft dysfunction and reduced need for mechanical circulatory support after transplant."
The SherpaPak is a sterile, single-use, temperature and pressure-controlled container for organ transport. The box closely maintains the temperature of the donor heart between 4°C and 8°C and is the only organ transport system with U.S. Food and Drug Administration and CE mark approvals. The heart is mechanically isolated in a leakproof canister that provides a consistent thermal environment for >40 hours and protects against physical and thermal trauma.
When to Refer to an AHF Specialist
Managing patients with AHF requires a number of critical decisions, but often the most critical one is made by the referring physician.
"When the patients get to me usually the diagnosis of severe AHF is already made. My job is to confirm the heart is sick enough for me to transplant and the rest of the body is well enough to handle a transplant," says Kittleson.
It's rare that she'll find a referred patient not sick enough for her clinic, but it's common to see patients being referred too late. There is a magic "window of opportunity" where the heart is sick enough to warrant a transplant or MCS, but the rest of the body – namely the liver, kidneys and lungs – have not been so damaged by the HF that the patient is well enough to handle the transplant or not confer prohibitive risk, she notes.
"Any hospitalization for decompensated HF should be considered as a sentinel event," she says. "That patient has a 20% to 30% chance of dying in the subsequent year, and we should be thinking about next steps for that patient."
Similarly, defibrillator shocks, worsening kidney or liver function, and having to back off on the guideline-directed medical therapy because of hypotension are all red flags that should trigger a referral.
"Frankly, the big picture we need to be thinking about now is working harder to prevent the development of HF in the first place," adds Givertz. "If we could just get physicians, nurse practitioners and physician associates to adequately treat hypertension and treat risk factors and reduce the risk of ischemic heart disease, we could prevent a lot of HF," he adds.
Since the start of clinical use in 2018, the system has been used to preserve more than 1,500 hearts, including one that was transported a record distance of 1,579 miles from Alaska to Washington state, with a total ischemic time of more than seven hours.
The TransMedics Organ Care System (OCS), often called the "heart in a box," is another important innovation in organ preservation. It's about the size of a large rolling suitcase and perfuses a warm, beating donor heart with oxygenated blood as it is being transported. Early evidence suggests that the OCS keeps the heart in a near-physiological state.
"The OCS makes it such that there's basically no ischemic time, so the sky's the limit," says Michelle Kittleson, MD, PhD, FACC, from Cedars-Sinai Medical Center in Los Angeles, CA. "You can take a heart almost anywhere, even to Hawai'i, where you could not take a heart before, because now you don't have to worry about that four-hour clock ticking." Cost is likely to be an issue though, she notes.
New Allocation Criteria (But Some Unintended Consequences?)
The United Network for Organ Sharing (UNOS) in 2018 implemented new adult heart and heart-lung allocation criteria aimed at prioritizing the sickest patients and reducing waitlist mortality, while enhancing geographic organ sharing and improving organ distribution equity.
The new allocation system moved from a three-tiered system to a six-tiered system, with the use of extracorporeal membrane oxygenation (ECMO) and nondischargeable biventricular assist devices (VADs) defining the patients at highest risk.
"Using the previous criteria, the same priority was given to patients on ECMO, balloon pumps or on inotropic support with hemodynamic monitoring. This was unfair because a patient on ECMO is much sicker than a patient on a balloon pump," says Kittleson. The updated criteria addressed this inequity.
Wait times and outcomes in the updated UNOS system have been examined in >50 papers. The results have been mixed, with some early studies showing a decrease in waiting time but also a decrease in post-transplant survival. More recent analyses have shown comparable survival.
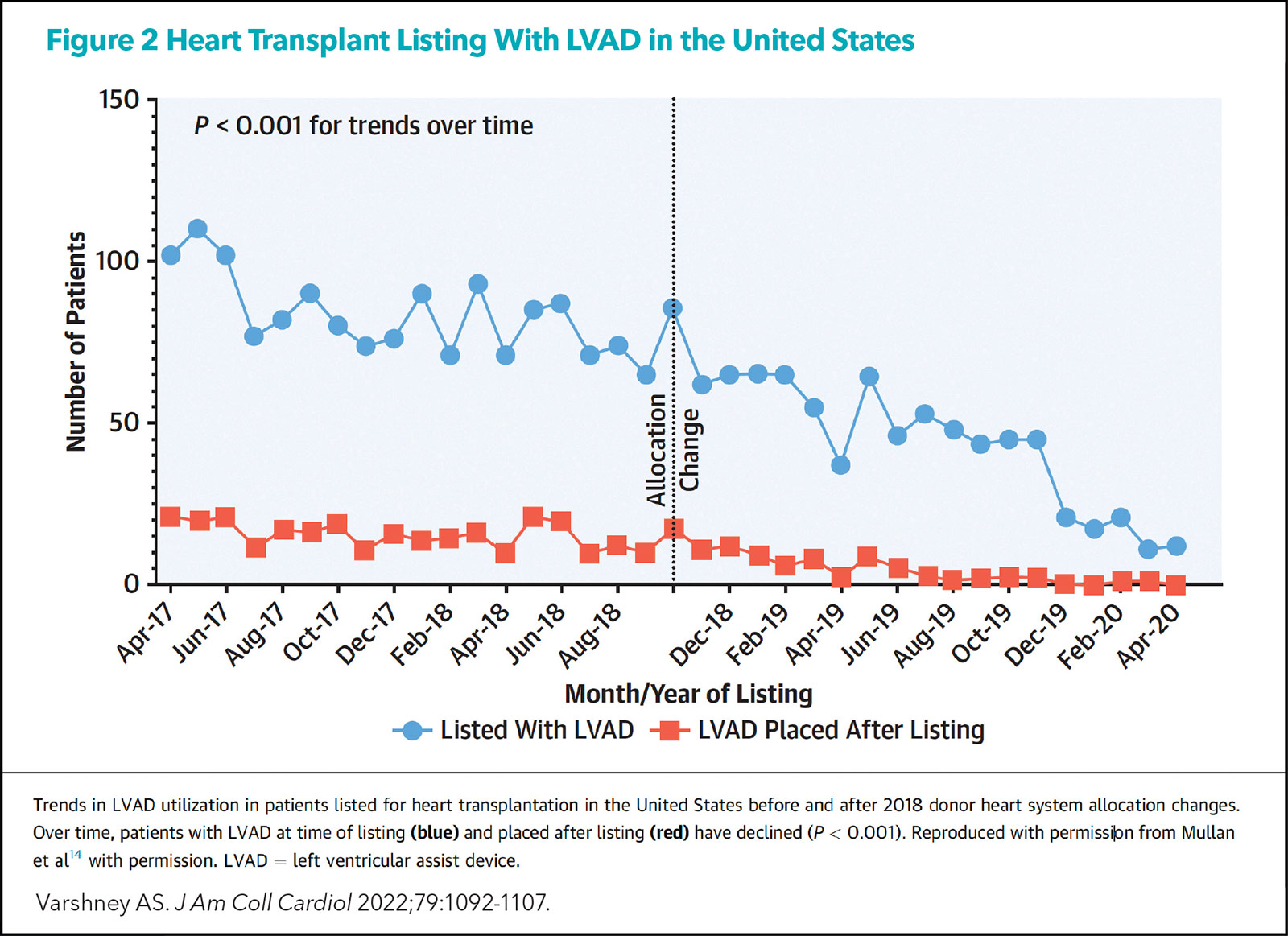
What has been seen clearly is an increased use of temporary mechanical circulatory support (MCS; by 20% to 30%) and a clear decrease in the numbers of patients listed with left VADs (LVADs) or in whom LVADs are being placed after listing (Figure 2).
Discussions are ongoing in the field about whether the system changes are a good thing or a bad thing, but a survey conducted by DeFilippis and colleagues showed that 84% of the 120 heart failure cardiologists and surgeons reported increasing their use of temporary MCS to achieve a higher UNOS status, and 70% believe the new system could harm patients though the overuse of temporary MCS.3
"The new system prioritizes patients who are supported by any temporary MCS, which has resulted in a lot of these patients spending more time in the hospital, often in the intensive care unit, on machines that carry the risk of complications including bleeding, hemolysis, infection and stroke," says DeFilippis. She adds the data suggest the devices are perhaps being overused because of the allocation criteria.
"We used to see more people waiting at home and they'd just come in for transplant, but that is less common now," DeFilippis tells Cardiology. "The patients who are getting transplanted are more often the sickest of the sick."
The challenge, suggests Kittleson, arises when a patient is getting worse on, say, inotropic support and could either be placed on temporary MCS and stay in the hospital waiting for a transplant or be implanted with an LVAD and go home.
"Even before the new criteria, we at Cedars-Sinai were very aggressive in moving a patient towards transplant. We would often give the patient a balloon pump or an Impella and take our chances waiting for a heart." She explains that other programs around the country, especially smaller ones where wait times for a heart may be months, might opt to place the patient on an LVAD.
Now that a patient with an LVAD at home has been moved down to Status 4 in the UNOS system (vs. Status 1B), it is not surprising to Kittleson that more patients are being put on temporary MCS. "Medically it makes sense to me to move a patient who is sliding down on inotropic support to temporary MCS, stabilize them and get them to transplant," she says.
"The problem is that putting patients on MCS too early undermines the allocation system," adds Kittleson. At Cedars-Sinai, policy dictates before an Impella or balloon pump can be considered, inotropic support must have first failed.
The issue was described candidly a few months ago in a JACC: Heart Failure editorial entitled "UNOS Left Me in Purgatory." The letter, written by a patient from East Islip, New York, describes how he received an LVAD in May 2016, with the understanding it would be a bridge to transplant. However, he has since become a Status 4 transplant candidate under the new allocation criteria because he has not experienced LVAD complications.
The allocation criteria will likely need revision, suggests Michael M. Givertz, MD, FACC, at Brigham and Women's Hospital and Harvard Medical School in Boston, MA. But the criteria are, in fact, performing as expected, with the increase in temporary MCS not surprising. "Patients on ECMO and other MCS devices are moving quickly to transplant in most regions of the country. I believe the new criteria have prevented some of the 'gaming of the system' seen previously." However, he notes, there may be a need for a revision to account for the length of time a person is on an LVAD and then the status is changed to a higher level. More data are needed before this or other adjustments are made.
Part of the early enthusiasm for durable LVADs was sparked by early reports that showed high rates of myocardial recovery and subsequent device explantation using combined LVAD support and aggressive adjuvant reverse remodeling drug therapies. That prospect has dimmed in recent years.
"It's not what anyone had hoped for," says Givertz. "In a very small number of hands, with a very focused recovery protocol like in the RESTAGE-HF study, the rates of myocardial recovery reached as high as 50%." But it was about 1% in the overall STS INTERMACS experience with durable MCS."
"When a young person comes in with new-onset HF or cardiogenic shock, there are still ongoing debates in the field as to the best approach to this patient. Should we rush to transplant or try to give his or her natural heart function a chance to recover either with or without an LVAD? Both an LVAD and transplant carry different sets of potential complications," says DeFilippis.
DCD donation
In 1967, Christiaan Barnard, MD, performed the first human heart transplant. The donor was a donation after cardiac death (DCD) donor, located in the operating room adjacent to the recipient. Ethical controversy associated with DCD donation led to the Harvard criteria for brain death in 1968 and the standard for donation became donation after brain death (DBD).
In DCD organ retrieval, criteria for brain death have not been met, but donors have sustained devastating neurologic injury with little hope for recovery. Outcomes from DCD donation, which is being increasingly used in Europe and Australia, are promising, with no differences in outcomes compared with DBD organ retrieval.
As the number of potential DBD donors has decreased over time, due to changes in the treatment of traumatic brain injury and intracranial hemorrhage, DCD donation is being reconsidered as a means of increasing the numbers of donor hearts.
How much increased acceptance and adoption of DCD donation will actually increase numbers is unclear. One recent article suggested it could increase the number of donor hearts available for transplant by 300 additional heart transplants a year in the U.S.4 Another suggested it could boost transplants by 30%, which would, at current numbers, be more than 1,100 additional transplants.5
"I think the adoption of DCD donations may be driven by industry to some extent as it works to make it as successful as possible. But I think the 30% increase, at least in the short- and mid-term, is unlikely. Things don't tend to move that fast," says Givertz.
There is a real risk of public backlash with DCD donation, notes Kittleson. "It's a complicated process with a lot of variables. Some of which sound almost sci-fi – the stand-off time when waiting to see if the heart will reanimate and the question of whether to restart the heart while it's still in the body and transfer to ex vivo perfusion – making it challenging for even the wider cardiology world to understand all of it." She notes the potential for a "clickbait soundbite" if DCD starts happening more often, which could ignite further fear and mistrust in the medical system. "The optics are one reason that many medical centers haven't started DCD donations yet," she adds.
As for whether the trend towards more transplantation will continue, Givertz says it's really anyone's guess. "It's hard to predict where things will go. There has been a significant increase in donor hearts because the safety and efficacy of HCV-infected organs has been demonstrated. I suspect this increase is more than we'll see from DCD donation. Yet, the size of the donor pool is not a zero-sum game; some factors can expand it and others contract it, for example solving the opioid epidemic thus reducing the number of hearts coming from that source."
"There are a lot of new and exciting opportunities for our patients with advanced heart failure. I think over the next few decades these improvements in technology and advances in post-transplant care are really going to change the quality of life of our patients and long-term outcomes," says DeFilippis.
Inequity in Organ Allocation
Does having a systematic means of organ allocation alleviate health disparities and structural inequities? Unfortunately, it appears the answer is no, or not entirely at least.
Historically, Black patients received proportionally fewer heart transplants than Whites or other racial groups, but this gap has slowly closed over the years. The proportion of Black patients listed for transplant increased from 7% in 1987 to 25% in 2019, and the proportion of transplants increased from 5% in 1987 to 26% in 2019.6 Still, there appears to be at least some bias in clinicians' decision-making process for allocation of advanced HF therapies.7
Sharad Wadhwani, MD, MPH, and colleagues from the University of California, San Francisco, suggest that insurance coverage, or a lack thereof, may explain some of these differences.8
The Organ Procurement and Transplantation Network (OPTN) policy specifically prohibits allocation to be based on race, ethnicity or socioeconomic status. However, financial resources are factored into waitlist decisions as part of a general assessment of "transplant suitability." This allows transplant centers to, essentially, circumvent the prohibition noted above. And exclusions are not solely based on whether patients are insured, but can include an assessment of whether the recipient will be able to "afford" out-of-pocket, noncovered expenses associated with transplantation.
"I think this is a really good example of 'perfect is the enemy of good.' Ideally everyone should just get a heart whenever they need it. But we know from bitter, tragic experience that a patient who gets a transplant who cannot afford the medications afterwards or doesn't have caregivers to take them to an appointment is likely to die," says Kittleson.
"I see this as a health system issue, not a doctor issue. It's a system issue we need to figure out."
Wadhwani and colleagues suggest a handful of fixes, including developing incentives for transplant centers to offer transplantation and support post-transplant care for those with limited financial resources.
MCS 101
MCS devices can be broadly categorized as either durable or temporary. On the durable side, LVADs are, by far, the most commonly used.
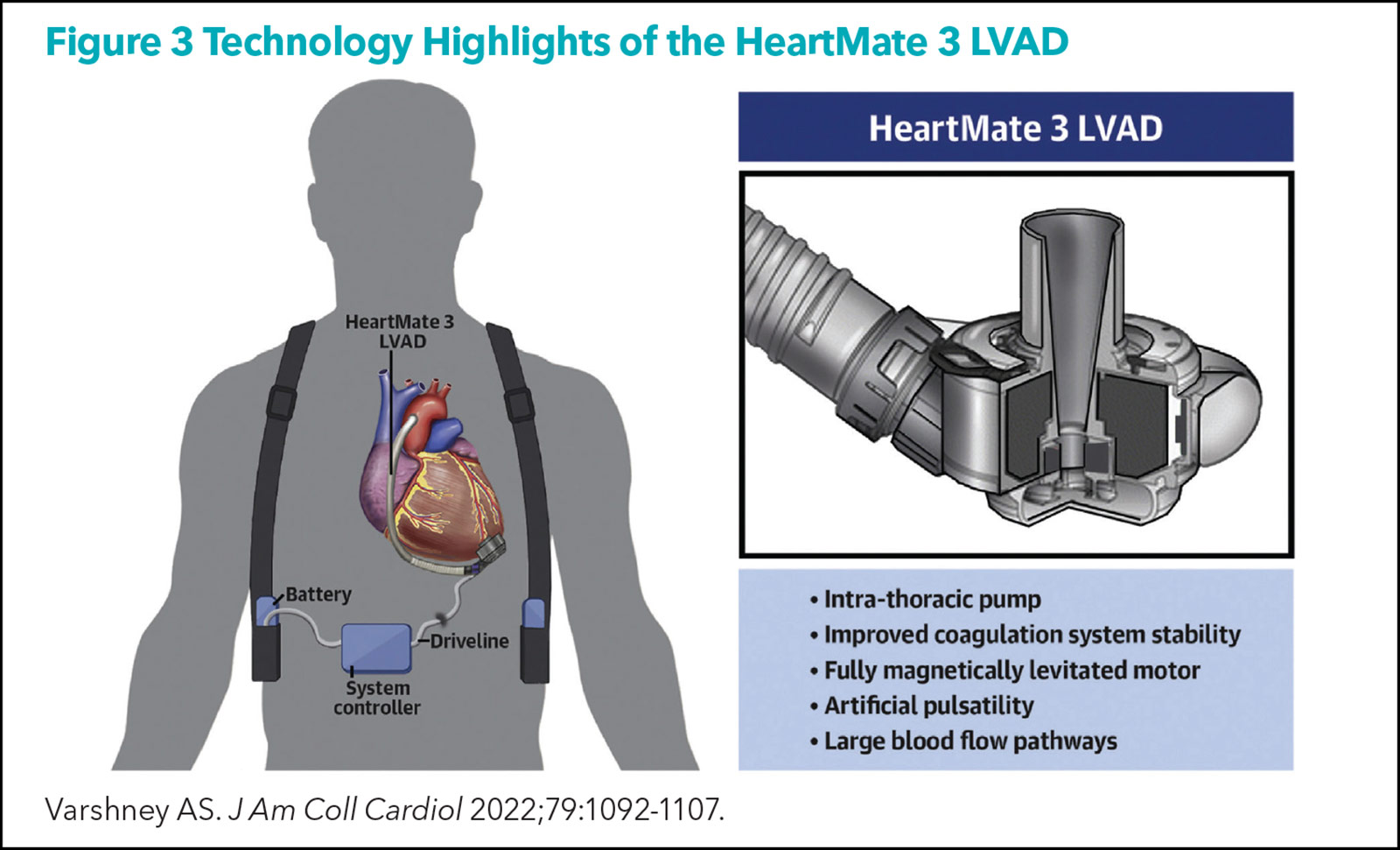
Since 2021 when Medtronic withdrew the HeartWare VAD from the market, the HeartMate 3 LVAD (Abbott; Figure 3) is the only LVAD commercially available in the U.S.
The migration of the field towards the HeartMate 3 started after the publication of the MOMENTUM 3 trial in 2019, which showed the superiority of the HeartMate 3 centrifugal flow device over the HeartMate II axial flow device.9 Although HeartMate 3 was never tested head-to-head against HeartWare, the fully magnetically levitated design and other novel features of HeartMate 3 resulted in almost complete elimination of pump thrombosis, one of the major complications of longstanding LV support.
Post-approval registries of HeartMate3 recipients show two-year survival of about 80%, "comparable to that seen with heart transplantation," notes DeFilippis. Average LVAD survival is expected to surpass five years in patients on durable HeartMate3 support.10
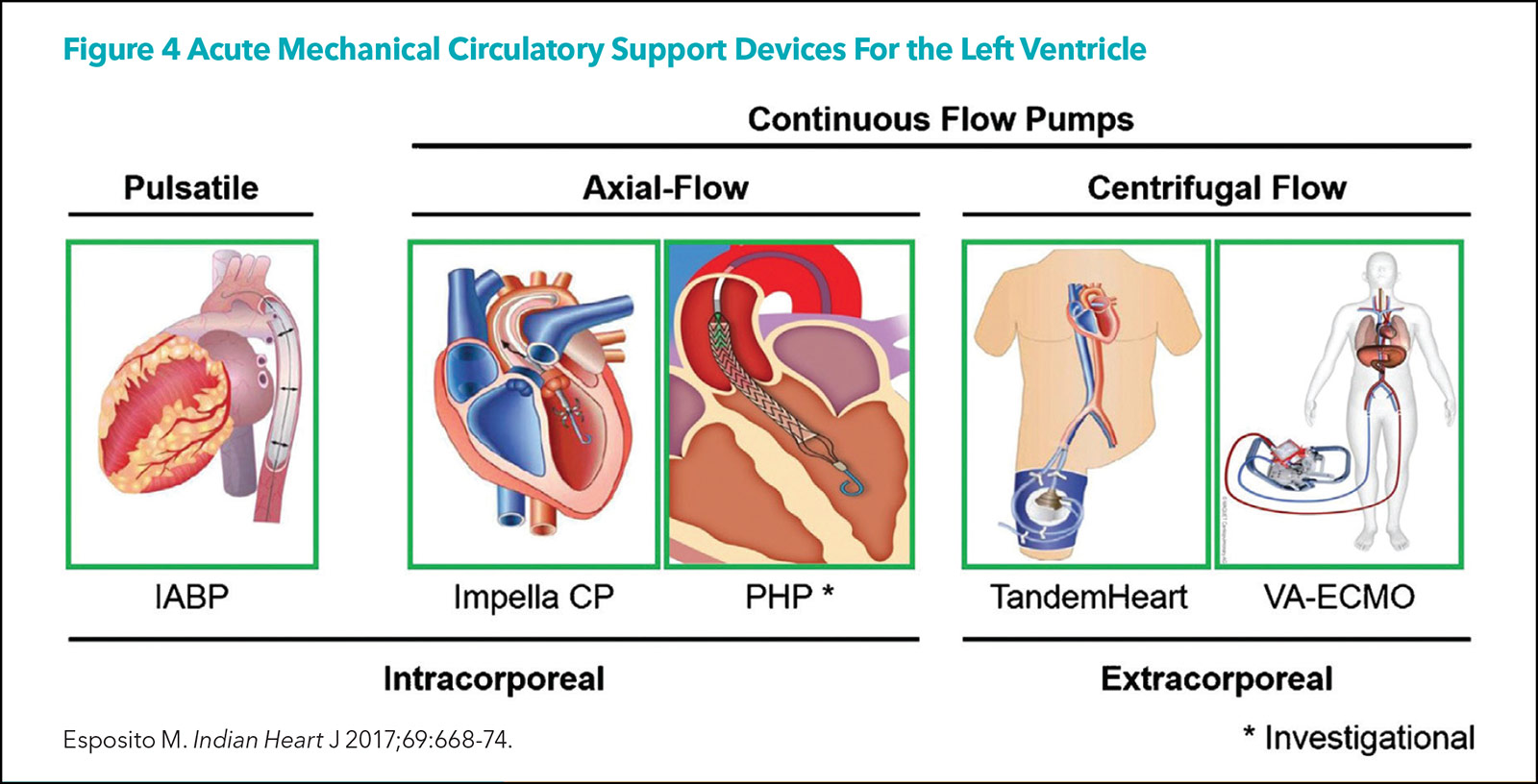
Temporary MCS to supplement cardiac output is most commonly used in the treatment of cardiogenic shock refractory to medical therapy. As such, it can serve as a bridge to recovery, a bridge to transplant, a bridge to durable LVAD implantation, or as palliative care. Importantly, it facilitates refined assessment of eligibility and provides additional time for decision-making.
There are a number of MCS devices available (Figure 4), but the three most common platforms are the intra-aortic balloon pump (IABP), the percutaneous VADs (Impella, TandemHeart, and others), and veno-arterial extracorporeal membrane oxygenation (VA-ECMO).
"CCUs today are full of patients with cardiogenic shock, sepsis, COVID pneumonia, acute renal failure, etc., thus temporary acute MCS devices are being used more often," says Givertz. "They work pretty well but have limitations in terms of duration of therapy and device-related complications, about which I don't think we hear enough."
This article was authored by Debra L. Beck, MSc.
References
- DeFilippis EM, Khush KK, Farr MA, et al. Evolving characteristics of heart transplantation donors and recipients: JACC Focus Seminar. J Am Coll Cardiol 2022;79:1108-23.
- Paragonix Technologies SherpaPak® Improves 1-Year Survival in Heart Transplant Patients in Real-World Study. Paragonix Technologies. Published April 28, 2022. Accessed May 12, 2022. Available here.
- DeFilippis EM, Psotka MA, Khazanie P, et al. Exploring physician perceptions of the 2018 United States Heart Transplant Allocation System. J Card Fail 2022;28:670-74.
- Madan S, Saeed O, Forest SJ, et al. Feasibility and potential impact of heart transplantation from adult donors after circulatory death. J Am Coll Cardiol 2022;79:148-62.
- Jawitz OK, Raman V, DeVore AD, et al. Increasing the US heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg 2020;159:e307-e309.
- Trivedi JR, Pahwa SV, Whitehouse KR, et al. Racial disparities in cardiac transplantation: Chronological perspective and outcomes. PLOS ONE 2022;17:e0262945.
- Breathett K, Yee E, Pool N, et al. Does race influence decision making for advanced heart failure therapies? J Am Heart Assoc 2019;8(22):e013592.
- Wadhwani SI, Lai JC, Gottlieb LM. Medical need, financial resources, and transplant accessibility. JAMA 2022;327:1445-6.
- Mehra MP, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 2019;380:1618-27.
- Varshney AS, DeFilippis EM, Cowger JA, et al. Trends and outcomes of left ventricular assist device therapy: JACC Focus Seminar. J Am Coll Cardiol 2022;79:1092-1107.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Prevention, Vascular Medicine, Implantable Devices, SCD/Ventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Acute Heart Failure, Heart Transplant, Mechanical Circulatory Support, Interventions and Vascular Medicine, Hypertension
Keywords: ACC Publications, Cardiology Magazine, Acute Kidney Injury, Adolescent, Analgesics, Opioid, Antiviral Agents, Brain Death, Brain Injuries, Traumatic, Cardiac Output, Cardiologists, Cardiology, Caregivers, Death, Defibrillators, Donor Selection, Duration of Therapy, Emblems and Insignia, Ethnic Groups, Extracorporeal Membrane Oxygenation, Gene Editing, Hazardous Substances, Heart Failure, Heart Transplantation, Heart Ventricles, Hemodynamic Monitoring, Hemolysis, Hepacivirus, Hepatitis B virus, Hepatitis C, Hepatitis C, Chronic, HIV Infections, Hospitalization, Hospitals, Hypertension, Hypotension, Incidence, Insurance Coverage, Intensive Care Units, Intracranial Hemorrhages, Kidney, Liver, Lung, Lung Transplantation, Operating Rooms, Opioid Epidemic, Organ Preservation, Palliative Care, Pandemics, Patient Care, Physicians, Plastics, Policy, Primary Graft Dysfunction, Quality of Life, Referral and Consultation, Registries, Risk Factors, Sepsis, Shock, Cardiogenic, Social Class, Stroke, Technology, Temperature, Thrombosis, Tissue and Organ Harvesting, Tissue and Organ Procurement, Tissue Donors, Universities, Waiting Lists, Heart-Assist Devices
< Back to Listings