Peripheral Matters | Transforming Acute PE Care: Standardizing Management to Improve Outcomes
Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is the third most frequent cause of cardiovascular death globally. Within the U.S. alone there are 900,000 cases of VTE annually and an estimated 200,000-250,000 related hospitalizations.1 The growth in device-based interventions coupled with the lack of a standardized treatment strategy has led to an opportunity to optimize management of acute PE using a multidisciplinary approach. This has primarily been through the creation of the Pulmonary Embolism Response Teams (PERTs) model.
How Has PE Been Treated?
The variability of clinical presentations in patients eventually diagnosed with PE range from asymptomatic to symptoms of right ventricular (RV) failure, and even with sudden cardiac death as a common first presentation.
The initial diagnostic approach to PE is a combination of history and risk stratification. The most used guidelines to aid in its management come from the American Heart Association (AHA) and European Society of Cardiology (ESC).2,3 Using predefined criteria based on vital signs, clinical imaging and laboratory findings, patients can be categorized as having a PE that is massive (AHA) or high risk (ESC); submassive (AHA) or intermediate risk (ESC); or low risk. The ESC guideline further stratifies intermediate risk into intermediate-high and intermediate-low; this delineation is based on both imaging presence of RV dysfunction and/or biomarker elevation.3 In addition, the Pulmonary Embolism Severity Index (PESI) and the simplified (sPESI) scoring systems risk stratify patients by estimating the 30-day all-cause mortality, while the AHA and ESC risk stratification goal is to identify those patients at highest risk of dying from PE within 30 days.
Current evidence shows that low-risk patients or patients without evidence of RV dysfunction treated with anticoagulation alone generally do well.3,4 Conversely, thrombolysis of high-risk PE has been proven lifesaving in emergent situations.5,8 Nonetheless, there remains a paucity of high-quality evidence for how to manage patients at intermediate risk or with a submassive PE. In this context, endovascular therapies such as catheter-directed thrombolysis (CDT) and mechanical thrombectomy (MT) are increasingly being used with limited randomized trial data. Currently, the goal of treatment with these devices is to improve short-term hemodynamics through reduction of thrombus burden, primarily among patients at high risk for an in-hospital event.7 The risk with these interventions, though infrequent, involve the balance of bleeding risk and need for operator experience.
The Value of PERT
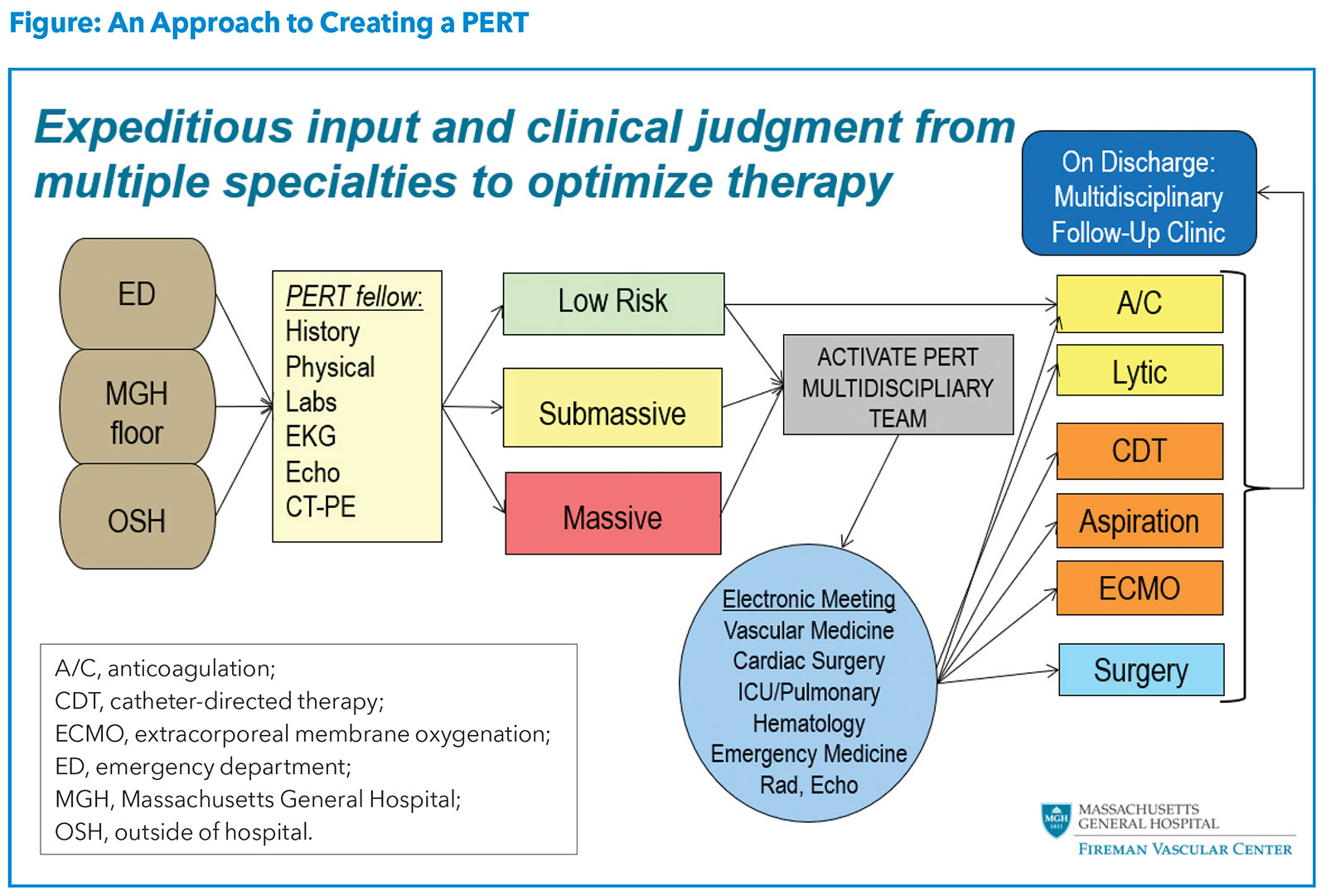
With the rise in the incidence and awareness of PE, the high associated morbidity and mortality, and the lack of a standardized treatment algorithm, there was a clear need for a strategic approach to caring for PE patients.9 This prompted the creation of a multidisciplinary approach termed PERT, which was developed using the heart team concept and rapid response systems as models. A sample algorithm for activation and operation of a PERT team is illustrated in the Figure.
The overall goal of PERT is to streamline coordination of care of the complex and/or high-risk PE patient using a multidisciplinary approach. Since the implementation of PERTs across multiple institutions in the U.S., studies have demonstrated how PERT has influenced treatment and outcomes among patients with PE. A study from the Massachusetts General Hospital showed that after implementing PERT, compared with before, patients were more likely to undergo advanced therapy without an increase in bleeding or a difference in mortality.10
One of the largest investigations of acute PE patients treated pre- and post-PERT implementation, from Beth Israel Deaconess Medical Center in Boston, found a reduction in the use of inferior vena cava filters in the post-PERT cohort; however, no change in PE-related mortality or bleeding was observed.11 Despite the lack of improvement in hard endpoints, the team-based approach has helped to improve appropriate adoption and utilization of device-based interventions.
Development of the PE Research Collaborative
Despite the increase in use of PERTs and more coordinated care for patients with PE, there remains a lack of universally adopted clinical definitions and endpoints among the various providers caring for these patients. This is coupled with the need for more prospective registry and trial data that capture meaningful data elements and outcomes. As a collaboration between the Food and Drug Administration (FDA) and the National PERT Consortium, the Pulmonary Embolism Research Collaborative (PERC) was created to tackle some of the major remaining issues and controversies existing in PE clinical care.
The inaugural meeting of PERC was held in Washington, DC, on April 22, 2022, and had two objectives: 1) create consensus on the data elements required in prospective registries and trials; 2) discuss controversial topics in the current management of PE. Topics included standardizing risk stratification, assessing the importance of thrombus burden, and the pre- and post-assessment of the right ventricular/left ventricular (RV/LV) ratio. The overall goal was not to develop guidelines or algorithms, but to engage in thoughtful discussion that would be able to clarify what is needed to best improve the practice and research of acute PE care. For example, RV dysfunction is currently being used as a primary endpoint for advanced therapies; however, this is based on older observational studies and there is a lack of evidence demonstrating that an improvement in RV/LV ratio after intervention is associated with reduced mortality. Another example is understanding the role and importance of anticoagulation in conjunction with advanced therapies.
Areas For Further Research
As we look ahead, two upcoming randomized controlled trials will help bridge the gap in the management of intermediate risk/submassive PE: HI-PEITHO and PEERLESS. HI-PEITHO aims to evaluate the treatment of acute intermediate high-risk PE with either the EKOS endovascular CDT system plus systemic anticoagulation vs. systemic anticoagulation alone. The primary endpoints are PE-related death, cardiorespiratory decompensation or collapse and symptomatic confirmed recurrence of PE.12 PEERLESS will compare CDT against MT with the FlowTriever system for the management of acute submassive PE. The primary powered outcome uses a win ratio approach to evaluate clinically meaningful endpoints. This trial also includes a nonrandomized cohort of 150 patients with an absolute contraindication to thrombolytics.13
The management of acute PE has undergone a transformation, driven by the rise of coordinated PERTs and the innovation in device-based therapies. As we look to the future, there remains a need for further standardization of the treatment approach, greater adoption of multidisciplinary PERTs and investment in obtaining prospective evidence. PERC is one initiative to help direct future PE care, coupled with critical ongoing clinical trials evaluating the role of advanced PE therapies.


This article was authored by Seanna Daves, MD, Clinical Vascular Medicine Fellow at Massachusetts General Hospital in Boston, and Eric A. Secemsky MD, MSc, FACC, director of Vascular Intervention in the CardioVascular Institute at Beth Israel Deaconess Medical Center in Boston, and section head of interventional cardiology and vascular research at its Smith Center for Outcomes Research in Cardiology at BIDMC. He is also assistant professor at Harvard Medical School. Reach out to him @EricSecemskyMD.
References
- CDC Fact Sheet. Blood Clots: A Serious but Preventable Medical Condition. Updated April 25, 2022. Accessed May 22, 2022. Available here.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-1830.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;49:315-52.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414-21.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: An international, open-label, randomized, non-inferiority trial. Lancet 2011;378:41-8.
- Giri J, Sista A, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: A scientific statement from the American Heart Association. Circulation 2019;140:e774-e801.
- Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv 2017;10:e004345.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016;133:98-103.
- Rosovsky R, Chang Y, Rosenfield K, et al. Changes in treatment and outcomes after creation of pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis 2019;47:31-40.
- Carroll B, Beyer SE, Mehegan T, et al. Changes in care for acute pulmonary embolism through a multidisciplinary pulmonary embolism response team. Am J Med 2020;133:1313-21.
- Konstantinides S, Rosenfield K. Ultrasound-facilitated, catheter-directed, thrombolysis in Intermediate-high risk pulmonary embolism (HI-PEITHO). NCT04790370. Available here.
- Wissam J, Gonsalves C, Stortecky S. The PEERLESS Study. NCT05111613. Accessed May 30, 2022. Available here.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Anticoagulation Management and Venothromboembolism, SCD/Ventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Interventions and Vascular Medicine
Keywords: ACC Publications, Cardiology Magazine, Algorithms, American Heart Association, Anticoagulants, Biomarkers, Cardiology, Catheters, Centers for Disease Control and Prevention, U.S., Consensus, Contraindications, Death, Sudden, Cardiac, Goals, Hemodynamics, Hospitalization, Hospitals, Incidence, Pulmonary Embolism, Reference Standards, Registries, Risk Assessment, Thrombectomy, Thrombolytic Therapy, Thrombosis, Treatment Outcome, United States Food and Drug Administration, Venous Thromboembolism, Venous Thrombosis
< Back to Listings