Peripheral Matters | The SAFE-IVC Study: Contemporary Practice Patterns, Clinical Outcomes Associated With Insertion and Retrieval of Inferior Vena Cava Filters

Inferior vena cava filters (IVCFs) for the prevention of venous thromboembolism (VTE) were first introduced in the 1960s, and their use has progressively increased over the past several decades. To date, an average of 30,000 to 40,000 IVCFs are inserted annually in the U.S.,1 even though current professional guidelines, such as those from the American Society of Hematology, the Society of Interventional Radiology and the Society for Vascular Medicine, all primarily recommend IVCF insertion only for secondary prevention of VTE in patients who have an active contraindication to or failure of anticoagulation therapy.2,3
Limited Efficacy Data on IVC Filters From RCTs
The PREPIC clinical trial, in 1998, was the first to investigate the role of permanent IVCFs as add-on therapies in addition to anticoagulation for the secondary prevention of VTE (i.e., a "belt and suspenders" strategy). After eight years of follow-up, this trial found a significant reduction in pulmonary embolism (PE) rates but no reduction in mortality, as well as a concurrent increase in recurrent deep vein thrombosis (DVT) (of which 45% was due to filter thrombosis) compared to anticoagulation alone.4,5 In 2015, the subsequent PREPIC2 trial studied retrievable IVC filters on top of anticoagulation, and found similar rates of PE and mortality at three months (when the IVC filters had to be removed per trial protocol), compared to anticoagulation alone.6
A separate trial in 2019 investigated the use of IVCFs for the primary prevention of VTE among young polytraumatized patients with absolute contraindications to prophylactic anticoagulation, and found no significant difference in death or PE compared to conservative therapy; however, there was benefit with IVCFs at reducing PE if anticoagulation could not be started after seven days of hospitalization.7
Taken together, these randomized clinical trials suggest that IVCFs do not provide a definitive benefit for primary or secondary prevention of VTE when systemic anticoagulation can be administered.
Increasing Safety Signals For IVC Filters from Post-Market Surveillance
While RCTs have not raised major concerns regarding the safety of IVCFs, the sample sizes (200 to 400 patients) were likely too small to observe infrequent but important complications of indwelling filters, including fractures, dislodgement, perforation, embolization and thrombotic obstruction resulting in caval thrombosis.
However, increased detection and report of these complications over the years eventually prompted the U.S. Food and Drug Administration (FDA) to issue safety communications in 2010 and 2014 to strongly encourage IVCF retrieval as soon as clinically feasible.8-11 As part of the 2014 communication, the FDA further mandated that manufacturers of IVCFs collect additional safety data from contemporary clinical practice. This resulted in the PRESERVE clinical registry, which included data from approximately 1,400 patients with IVCFs from 54 U.S. hospitals between 2015 and 2019. This single-arm study found a reassuring safety profile for IVCFs, with low a low rate of periprocedural adverse events at the time of insertion and retrieval (0.5%), and low complications over the long term, such as IVC perforation in 1.4% of the cases.12
While this well-done study informed contemporary practice among selected centers, the findings from the PRESERVE registry had limited generalizability due to its focus on highly selected urban tertiary care centers, which may not capture broader clinical practice. For example, almost half (49.3%) of retrievable filters in PRESERVE were removed within 12 months of placement – which does not match broader contemporary clinical practice, where filter retrieval rates have been estimated to be as low as 15-20%.13
Impetus and Rationale For the SAFE-IVC Study
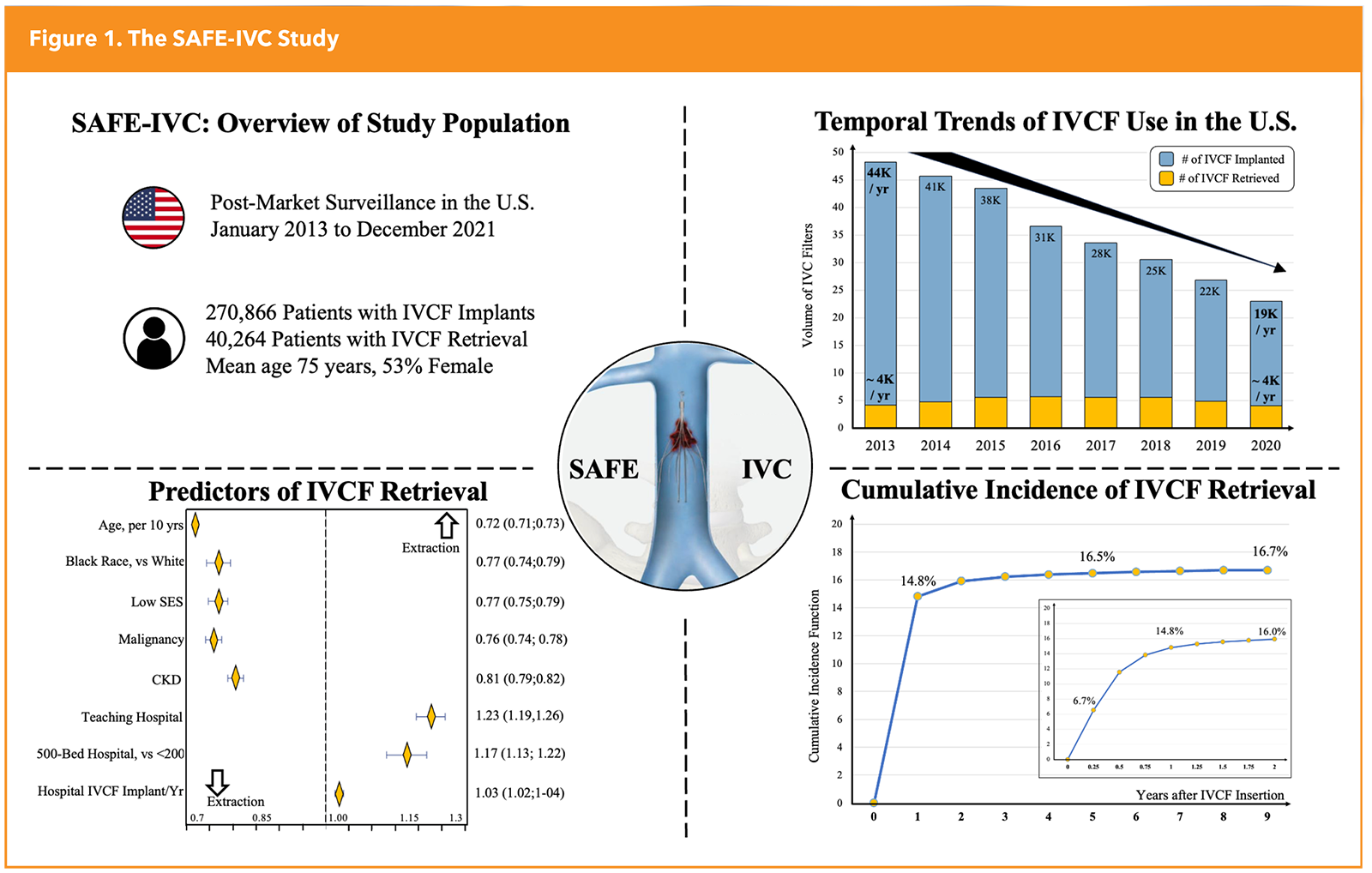
To better understand contemporary practice patterns and safety outcomes associated with the use of IVCFs, with an emphasis on generalizability, the SAFE-IVC study was designed through a collaboration between the FDA and the Smith Center for Outcomes Research at the Beth Israel Deaconess Medical Center in Boston (Figure 1).14
At the Smith Center, we leverage nationwide insurance claims databases and apply state-of-the-science analytical methods to characterize the real-world impact of cardiovascular drugs and devices. For this study, we aimed to: 1) describe temporal trends and contemporary practice patterns in IVCF insertion and retrieval among Medicare patients in the U.S., 2) identify patient- and hospital-level characteristics associated with filter retrieval, and 3) describe the incidence of periprocedural and long-term safety events throughout the clinical lifecycle of IVCFs (insertion, dwell time, retrieval). While the Medicare insurance plans primarily cover older patients aged >65 years, the Medicare population is actually the most directly affected by IVC filters, as nearly one every six Medicare beneficiaries with a PE receive a filter in the U.S.
Main Results of the SAFE-IVC Study
From 2013 to 2021, a total of 270,866 IVCF insertion procedures were performed by >16,000 operators across >3,800 hospitals in the U.S. These were followed by 40,264 retrieval procedures performed by approximately 8,500 operators across 1,800 hospitals. Therefore, in this broad nationwide cohort, we found a rather low retrieval rate at only 15%.
The annual volume of IVCF insertions decreased from about 45,000 per year in 2013 to about 20,000 per year in 2021, but the annual volume of retrieved IVCFs remained stable at about 4,000 per year during the entire study period. Older age and more comorbidities were associated with decreased likelihood of retrieval, while placement at large teaching hospitals were associated with increased likelihood of retrieval.
As expected for the Medicare population, patients undergoing IVCF insertion were older (mean age, 75 years, 53% female) and with a high burden of comorbidities. Notably, approximately 65% of patients received IVCFs to treat first-time VTE and approximately 25% for recurrent VTE, with very low use of IVCFs for VTE prophylaxis. Furthermore, about 65% of IVCFs were placed in patients who had major contraindications to anticoagulation, including about 50% with recent major bleeds and/or about 25% with major trauma. Overall, these findings suggest alignment between clinical practice and the aforementioned professional guidelines.
IVCF insertion was associated with a low risk of filter-related complications at 30 days (around 0.5%). Although the observed incidence of all-cause mortality at 30 days from insertion was disproportionately higher at approximately 14.5%, this was due to associated conditions, including bleeding and trauma, that served as the indication for IVCF insertion but also conferred a high short-term mortality risk.
Among the small subgroup of patients undergoing filter retrieval, most extraction procedures were performed within the first 12 months after insertion, and were successful in the vast majority (93.5%) of cases – with a low incidence of 30-day complications (around 1%). On the other hand, chronically indwelling IVC filters (>one year after insertion) were associated with about a 9% cumulative incidence of DVT requiring hospitalization and about a 2% incidence of caval thrombosis through nine years after insertion.
Unmet Need and Future Directions in the IVC Filter Space
Despite professional guidelines and FDA safety communications, the SAFE-IVC study found that IVCF retrieval rates remain low at 15%, and that chronic indwelling filters are associated with long-term complications.
As such, dedicated initiatives should be implemented to systematically generate a filter disposition plan at the time of insertion. In a minority of cases, the indication for filter insertion (i.e., prothrombotic state secondary to malignancy) or the contraindication to anticoagulation (i.e., spontaneous intracerebral hemorrhage) may justify permanent filter use. However, in most other cases, IVCF retrieval should be planned and executed as soon as clinically feasible. Several opportunities exist to help achieve this goal. For instance, computerized reminders embedded within the electronic medical records have been shown to nearly double filter retrieval rates.15 In addition, opportunistic screening softwares, powered by artificial intelligence, may help identify indwelling IVC filters on routine imaging. These and other tools can be used to continuously reevaluate indications for ongoing filter use and to ensure that filter insertions and retrievals are based on guideline recommendations.


This article was authored by Enrico G. Ferro, MD, cardiac electrophysiology fellow at Beth Israel Deaconess Medical Center (BIDMC), Harvard Medical School (HMS), and research fellow at the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, and Eric A. Secemsky, MD, RPVI, MSc, FACC, director of Vascular Intervention at BIDMC and section head of Interventional Cardiology and Vascular Research at the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at BIDMC, and associate professor of medicine at HMS, all in Boston.
References
- Ahmed O, et al. Rising retrieval rates of inferior vena cava filters in the United States: Insights from the 2012 to 2016 summary Medicare claims data. J Am Coll Radiol. 2018; 15:1553-57.
- Ortel TL, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693-38.
- Kaufman JA, et al. Society of Interventional Radiology clinical practice guideline for inferior vena cava filters in the treatment of patients with venous thromboembolic disease. J Vasc Interv Radiol. 2020;31:1529-44.
- Decousus H, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338;409-15.
- Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416-22.
- Mismetti P, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313:1627-35.
- Ho KM, et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019;381:328-37.
- Nicholson W, et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170:1827-31.
- Kadakia KT, et al. Information disclosure, medical device regulation, and device safety: the case of Cook Celect IVC filters. Ann Intern Med. 2024;177: 1711-18.
- US Food and Drug Administration.Removing Retrievable Inferior Vena Cava Filters: Initial Communication. August 9, 2010. Accessed June 7, 2024. Available here.
- US Food and Drug Administration. Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication. May 6, 2014. Accessed June 7, 2024. Available here.
- Johnson MS, et al. Predicting the safety and effectiveness of inferior vena cava filters (PRESERVE): outcomes at 12 months. J Vasc Interv Radiol. 2023.
- Rojas-Hernandez CM, Zapata-Copete JA, García-Perdomo HA. Role of vena cava filters for the management of cancer-related venous thromboembolism: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2018;130:44-50.
- Ferro EG, et al. Postmarketing surveillance of inferior vena cava filters among US Medicare Bbeneficiaries:The SAFE-IVC Study. JAMA. 2024;332:2091-2100.
- Mikhael B, et al. Usefulness of a computerized reminder system to improve inferior vena cava filter retrieval and complications. Am J Cardiol. 2019;123:348-53.
Clinical Topics: Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine
Keywords: Cardiology Magazine, ACC Publications, Venous Thromboembolism, Vena Cava Filters