Peripheral Matters | Peripheral Chronic Total Occlusion: Developing Strategies, Knowledge Sharing
Chronic total occlusions (CTO) are important anatomic variants for interventional cardiologists. Early in our training, we are forced to recognize the impact of total occlusion of a coronary vessel as it pertains to clinical decision-making, procedural risk and technical planning.
Interventional cardiologists invested in the peripheral vascular space have brought many elements of our coronary experience to the treatment of vascular disease. We would never deny our clinical cardiac expertise to a patient simply because their initial presentation to our clinic was with a noncardiac vascular condition. Similarly, we strive to bring the technical experience we gained from our work in the coronary bed to all our work in the periphery. It is with this mindset that we continue our ongoing evaluation and treatment of peripheral CTO.
Any responsible comparative discussion of coronary and peripheral CTO must begin by stressing the significant differences between these pathologies.1 The most important difference is the sequela of treatment complications. The risks of CTO PCI, even in the hands of experienced CTO PCI operators are very real. Compared with non-CTO PCI, CTO PCI requires greater contrast volume, longer fluoroscopy time and significantly higher risks of myocardial infarction, transfusion, cardiac tamponade, stroke, urgent cardiac surgery and death.2
Treatment of coronary and peripheral CTO carries many of the same anatomic risks including dissection and perforation. However, the clinical results of these anatomic risks are dramatically different. Wire perforation in the superficial femoral artery (SFA) may be an annoyance without clinical sequelae. The same wire perforation in a coronary artery could result in tamponade and emergent death.
Even in experienced hands, coronary CTO PCI carries a major adverse cardiovascular and cerebrovascular event (MACCE) rate of 7 percent and a 0.9 percent risk of death.3 For this reason, the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention recommended (Class IIa) PCI of a CTO in patients with appropriate clinical indication and anatomy only when "performed by operators with appropriate expertise."4
A EuroCTO Club consensus document further suggested that "retrograde techniques should be reserved for very experienced antegrade operators (>300 CTOs and >50 per year)."5 On the contrary, in the peripheral space, device trials have demonstrated a prevalence of SFA CTO as high as 20-30 percent in patients deemed clinically appropriate for revascularization.6,7
With such high rates of CTO encounter, and with relative safety of cautious procedural performance, it is often argued that for an interventional cardiologist to take on the responsibility of working in the vascular space, they should feel comfortable working on peripheral CTO.
Over a decade ago, the TASC II guidelines classified peripheral stenosis by presence of CTO and length and location. This work also took the important step of predicting anatomies favoring surgical vs. endovascular repair.8 As time has progressed, so too have our available tools and technical strategies. Our characterization of peripheral CTO has grown more sophisticated in response to increasing mechanisms for CTO crossing and repair and our desire for algorithmic decision-making improvements.
Saab, et al., recently developed the CTOP classification, based on data from the retrospective Chronic Total Occlusion Crossing Approach Based on Plaque Cap Morphology trial.9 This classification not only characterized CTO via its cap morphology, but offered insight into differential treatment of CTO based on that morphology. Four types of cap morphology were specified with types I-IV increasing in complexity:
- Type I with both concave proximal and distal caps.
- Type II with concave proximal cap and convex distal cap.
- Type III with convex proximal cap and concave distal cap.
- Type IV with both convex proximal and distal caps.
The foundational premise behind the utility of the CTOP classification is that cap characterization directs device passage into the CTO either through the native lumen or via the subintimal plane. Proximal and distal cap characterization can thus be utilized to select crossing strategy (antegrade vs. retrograde vs. combined).
The authors concluded that CTOP type I lesions were the easiest to cross via the antegrade approach and type IV lesions were the most difficult. They also demonstrated that CTOP types II, III and IV benefited from the addition of retrograde tibiopedal access.
An algorithmic approach to the percutaneous treatment of the coronary CTO was elegantly delineated by Brilakis, et al., in 2012.10 Maeremans and colleagues examined the real-world application of this coronary CTO "hybrid algorithm" in the RECHARGE registry data in 1,177 patients in Europe who underwent a total of 1,253 elective CTO PCIs between January 2014 and October 2015. This work demonstrated overall "hybrid algorithm" procedure success of 86 percent and major in-hospital complications of 2.6 percent.11
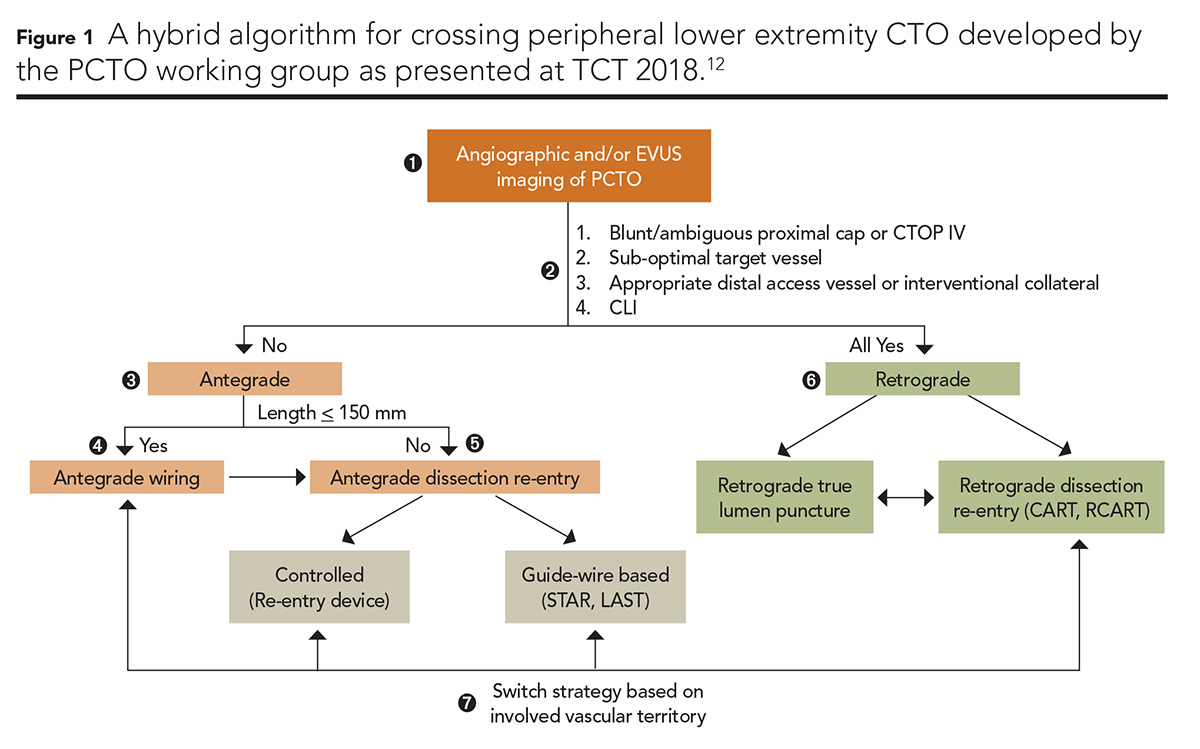
A PCTO Working Group composed of nine interventional cardiologists, one vascular surgeon and one interventional radiologist, and led by Bannerjee and colleagues, has recently taken on the similar challenge of delineating an evidence-based algorithmic approach to tackling peripheral CTO in the lower extremities (Figure 1).12
This sophisticated proposal combines analysis of CTOP classification with distal and collateral vessel analysis with clinical indication for revascularization to guide peripheral operators through the decision-making for treatment of peripheral CTO. The algorithm selectively recommends antegrade, retrograde and hybrid techniques as well as strategies for the performance of each. It represents a heroic advance in our approach to the treatment of peripheral CTO.12
Within each of the pathways (antegrade, retrograde, hybrid) there are many distinct crossing tactics which can be utilized and their distinct effectiveness should be studied further. The coronary literature has "named" many of these tactics (such as STAR, Mini-STAR, LAST, etc., explained later). Peripheral operators use many (though not all) of the same techniques, but we discuss them (and thus teach them) less frequently in the peripheral CTO literature.
In the periphery, the relative ease, safety and omnipresence of retrograde access opportunities may have made us more reliant on retrograde wire passage and thus less interested in defining, developing and studying complex crossing tactics. Indeed, many of us have become very willing to puncture diseased or even calcified occluded distal tibial vessels to attack a refractory femoral CTO from below.
In contrast, even an experienced coronary operator is less willing to attack a refractory right coronary artery CTO via "septal surfing" a retrograde approach that requires passage through a moderately diseased left main trunk. Rather than progressing through named strategies, failed peripheral antegrade cap puncture often leads to an increasingly rapid decision to "stick the foot" and "go retro."
Use of advanced crossing tactics begins with improved fundamental understanding of wire characteristics including penetrability, pushability, trackability, bending and lubricity.13 The correct technique in the correct situation fails with the wrong wire. Though utilized frequently in peripheral procedures, guidewire "handling" techniques are also more extensively described in the field of coronary CTO. These include:13
- "Sliding technique" of a Fielder FC (Asahi Intecc) wire being "slid" through younger, softer tissue
- "Drilling technique" with a fast but controlled 360-degree rotation with a high tip-load non-tapered wire with only gentle advancement "akin to drilling a bore-well"
- "Penetrating technique" of smaller 45-90 degree rotations with higher penetration force akin to "turning a screw"
- Modern "push and torque" technique utilized with the Gaia (Asahi Intecc) series of wires which involves pushing the wire with force but only gentle rotation and awaiting the wire to achieve the provided torque.
The antegrade approaches utilizing these wire techniques are further categorized into:
- Single wire techniques focused on finding and surfing small, invisible microchannels tracking through soft tissue
- Two wire techniques like the parallel wire technique in which a first wire is passed, occluding a patent channel and delineating the vessel course, but a second wire is utilized to find a potential new path to successfully cross the occlusion
- Subintimal tracking techniques (see below)
- Device-assisted tracking which describes antegrade crossing typically in the coronaries with the Crossboss and Stingray system (Boston Scientific).
The subintimal tracking techniques can be further characterized as subintimal tracking and reentry (STAR), introduced by Colombo, et al., in which an "umbrella-handle shaped" bend at the tip of a hydrophilic wire (more commonly now referred to as a "knuckle wire") is passed within the dissection flap, thus distributing force over the larger surface area of the bend to break through the subendothelial layer.14,15
Carlino, et al., introduced contrast-guided STAR in which contrast is injected into the subintimal space via a microcatheter to enlarge, connect and delineate microchannels available for crossing.16 Galassi, et al., further refined STAR by introducing "Mini-STAR" which uses the soft polymer fielder wires with support from a microcatheter with the goal of creating smaller subintimal spaces because of the smaller knuckle created around the microcatheter.17
The limited antegrade subintimal tracking (LAST) technique introduced by Thompson, et al., is similar but uses stiff "penetration" wires to redirect into the distal true lumen after advancement with the other techniques described above.18,14 Variants of these techniques are used commonly in the peripheral space though peripheral operators rarely utilize this nomenclature.
In that the peripheral space offers a tremendous amount of equipment options (even including platforms ranging from 0.014" to 0.035" wires), perhaps if we analyzed these strategies in peripheral interventions more closely we could at least optimize what devices to use for which techniques.
Finally, combinations of these techniques have been utilized in the coronary space to obtain strategic goals as a step in antegrade CTO crossing. An example is the "move the cap" techniques in which an ambiguous proximal coronary cap prevents direct passage through the CTO cap.
In that setting "penetrating" wires can be used to enter the subintimal space proximal to the ambiguous termination of the coronary proximal occlusion. The "scratch-and-go" technique can then be used to advance equipment through the subintimal space (using combinations of the above strategies).
Balloon-assisted subintimal entry (BASE) can also be performed in which angioplasty is utilized to create microchannel entrance to the subintima proximal to the nebulous cap thus effectively "moving the cap" proximally.19
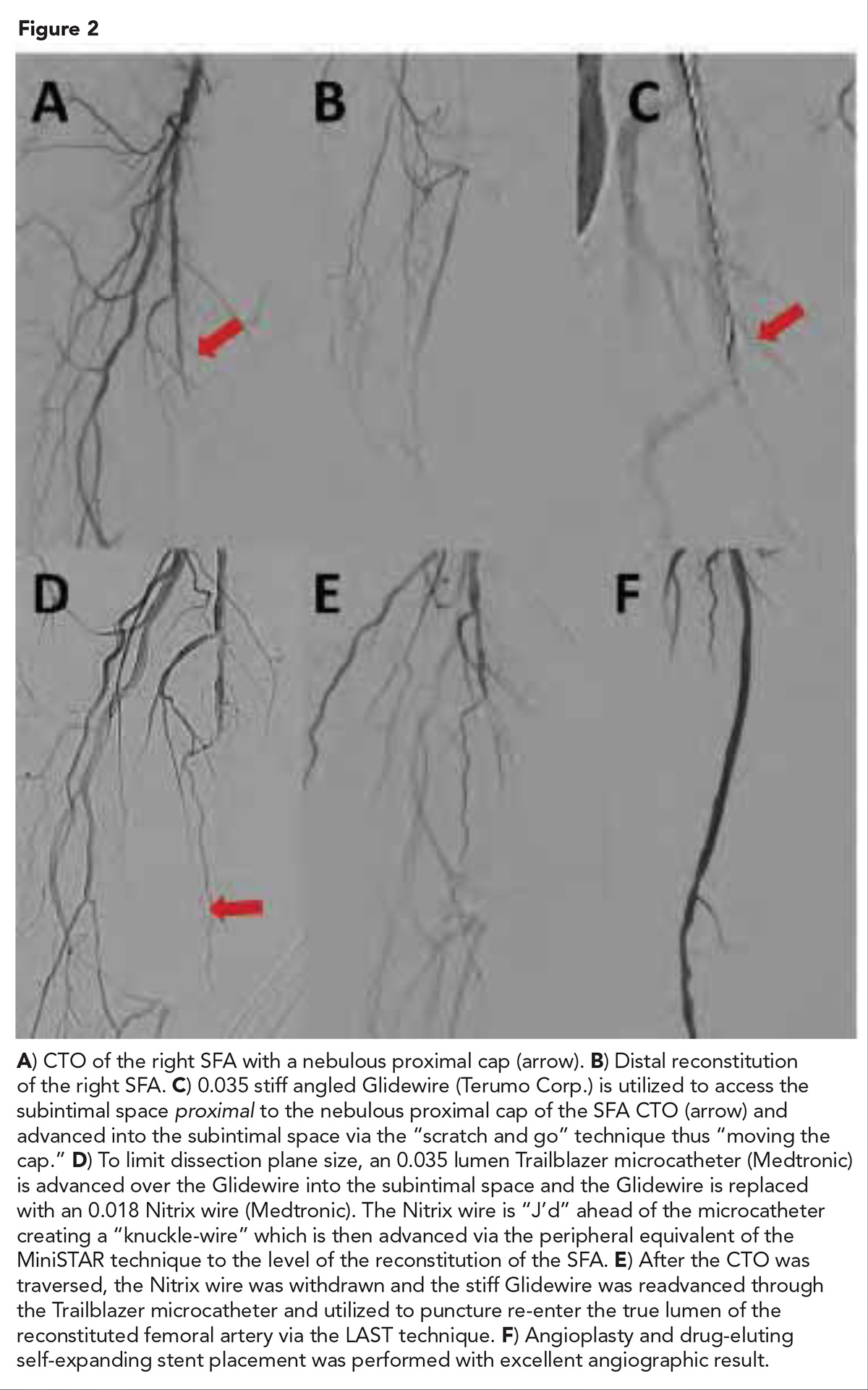
Figure 2 presents an example of "moving the cap" in a peripheral superficial femoral artery CTO from our lab in Austin, TX. This patient presented with lifestyle limiting claudication symptoms preventing his ability to continue his work.
Angiography demonstrated total occlusion of the right SFA with a nebulous proximal cap and clear distal SFA reconstitution. The patient had severe below knee disease with one vessel runoff via a diseased residual AT. The nebulous proximal cap complicates antegrade crossing. Severe below knee disease increased the risk of retrograde strategies.
Applications of strategies from the coronary literature including "scratch and go," "MiniSTAR" and "LAST" were utilized to cross the CTO resulting in an excellent angiographic result. With femoral inflow corrected, the patient's claudication symptoms resolved completely post procedure and he returned to work.
We should discuss and teach this knowledge base and these techniques to peripheral operators more frequently and actively. They were not necessarily a component of the training of every vascular surgeon or interventional radiologist like they were part of our interventional cardiology training. So it is our responsibility to advance them within the peripheral field. Knowledge of and ability with them opens a number of interesting doors in peripheral CTO.
First, some peripheral cases lack anatomic opportunities for retrograde access and therefore strategies aimed at optimizing antegrade success are extremely valuable. We do not always need an expensive crossing device and in some cases these techniques may outperform device-assisted crossing.
Second, many operators remain uncomfortable with retrograde strategies in claudicants because of desire to avoid distal complications which could convert the relatively benign natural history of claudication into a more severe disease through distal complication.
The PCTO Working Group's peripheral CTO hybrid algorithm acknowledges a heightened willingness to transition to retrograde in critical limb ischemia (vs. claudication) peripheral CTO cases.12 Therefore, advanced thoughtful antegrade strategy escalation may be particularly useful in claudicants.
Third, the larger sizes of access sheaths and vessels in the periphery and the multiple available peripheral platforms create a wide variety of combinations of devices for use in these strategies. We should evaluate and optimize device utilization in the various techniques.
Fourth, because of the relative safety of retrograde device maintenance in the periphery (whereas coronary CTO retrograde devices can induce myocardial ischemia or can cause dreaded septal injury) and because of the larger caliber of retrograde peripheral vessels (especially when retrograde femoral or popliteal access is obtained), each of these strategies can be used both for peripheral antegrade and peripheral retrograde passage of the CTO if the retrograde cap is not amenable to direct wire crossing.
Therapies and technical treatments for peripheral artery disease (PAD) and peripheral CTO are increasing and improving exponentially. Importantly, leaders in the vascular space have taken on the challenge of developing algorithms for strategic implementation and evaluation of these techniques in treatment of peripheral CTO (such as the CTOP Classification and the PCTO Working Group hybrid treatment algorithm).
One of our greatest advantages in our treatment of PAD is our opportunity to attack it with a multidisciplinary approach with surgeons, radiologists, vascular medicine doctors and cardiologists each bringing distinct subspecialty knowledge to addressing PAD. The robust coronary CTO experience is one of many tools that we cardiologists will continue to leverage in the ongoing battles ahead.
We peripheral CTO operators should use what coronary CTO operators have learned and we should be sure to collaboratively advance both fields through ongoing knowledge sharing. This is a great opportunity for collaborative foundational knowledge to continue to advance multidisciplinary care as well as for our field to optimize our battle with PAD.

This article was authored by Peter P. Monteleone, MD, FACC, at the University of Texas at Austin Dell School of Medicine, Seton Heart Institute, in Austin, TX.
References
- Banerjee S. Circulation 2016;134:901-3.
- Brilakis ES, Banerjee S, Karmpaliotis D, et al. JACC Cardiovasc Interv 2015;8:245-53.
- Sapontis J, Salisbury AC, Yeh RW, et al. JACC Cardiovasc Interv 2017;10:1523-34.
- Levine GN, Bates ER, Blankenship JC, et al. J Am Coll Cardiol 2011;58:e44-122.
- Sianos G, Werner GS, Galassi AR, et al. EuroIntervention 2012;8:139-45.
- Schneider PA, Laird JR, Tepe G, et al. Circ Cardiovasc Interv 2018;11:e005891.
- Dake MD, Ansel GM, Jaff MR, et al. Circulation 2016;133:1472-83.
- Norgren L, Hiatt WR, Dormandy JA, et al. J Vasc Surg 2007;45 Suppl S:S5-67.
- Saab F, Jaff MR, Diaz-Sandoval LJ, et al. J Endovasc Ther 2018;25:284-91.
- Brilakis ES, Grantham JA, Rinfret S, et al. JACC Cardiovasc Interv 2012;5:367-79.
- Maeremans J, Walsh S, Knaapen P, et al. J Am Coll Cardiol 2016;68:1958-70.
- Banerjee S, Shishehbor M, Parikh M, et al. A percutaneous crossing algorithm for femoropopliteal and below-the-knee peripheral artery chronic total occlusions (PCTO algorithm). The PCTO working group. Presentation at TCT 2018.
- Mishra S. Indian Heart J 2017;69:266-76.
- Galassi A, Grantham A, Kandzari D, et al. Interv Cardiol 2014;9:201-7.
- Colombo A, Mikhail GW, Michev I, et al. Catheter Cardiovasc Interv 2005;64:407-11.
- Carlino M, Godino C, Latib A, et al. Catheter Cardiovasc Interv 2008;72:790-6.
- Galassi AR, Tomasello SD, Costanzo L, et al. Catheter Cardiovasc Interv 2012;79:30-40.
- Lombardi WL. J Invasive Cardiol 2009;21:543.
- Vo MN, Karmpaliotis D, Brilakis ES. Catheter Cardiovasc Interv 2016;87:742-8.
Clinical Topics: Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Pericardial Disease, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, Algorithms, Coronary Angiography, Angiography, Angioplasty, Bone Screws, Cardiac Tamponade, Constriction, Pathologic, Coronary Artery Disease, Coronary Vessels, Decision Making, Coronary Occlusion, Femoral Artery, Fluoroscopy, Goals, Hearing Loss, Sensorineural, Life Style, Lower Extremity, Myocardial Infarction, Percutaneous Coronary Intervention, Polymers, Peripheral Arterial Disease, Prevalence, Registries, Retrospective Studies, Stroke, Surgeons
< Back to Listings