Peripheral Matters | IVUS-Guided Peripheral Vascular Intervention
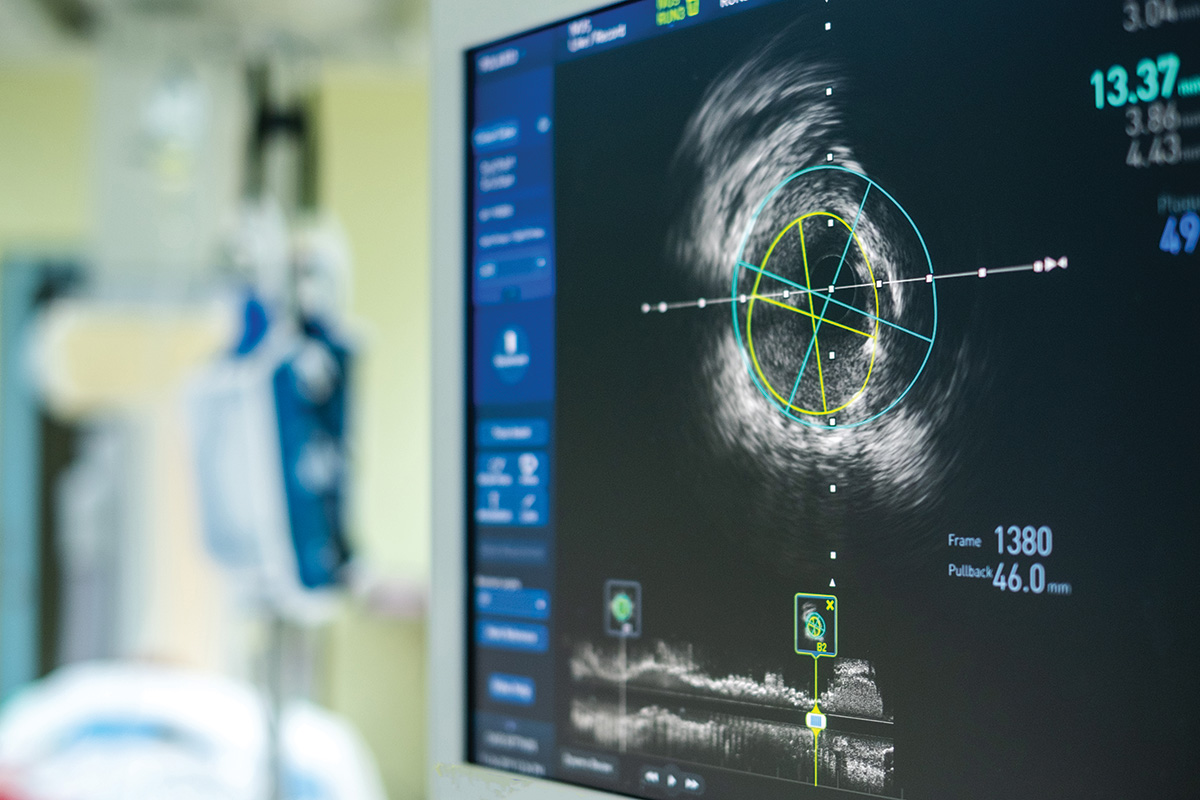
Intravascular ultrasound (IVUS), a technology developed in the 1970s, relies on piezoelectric transducers positioned at the end of intraluminal catheters that can produce high-quality cross-sectional imaging of the vascular lumen.
While angiography has been the primary modality used in peripheral vascular intervention (PVI), this technique has several major limitations compared with IVUS.
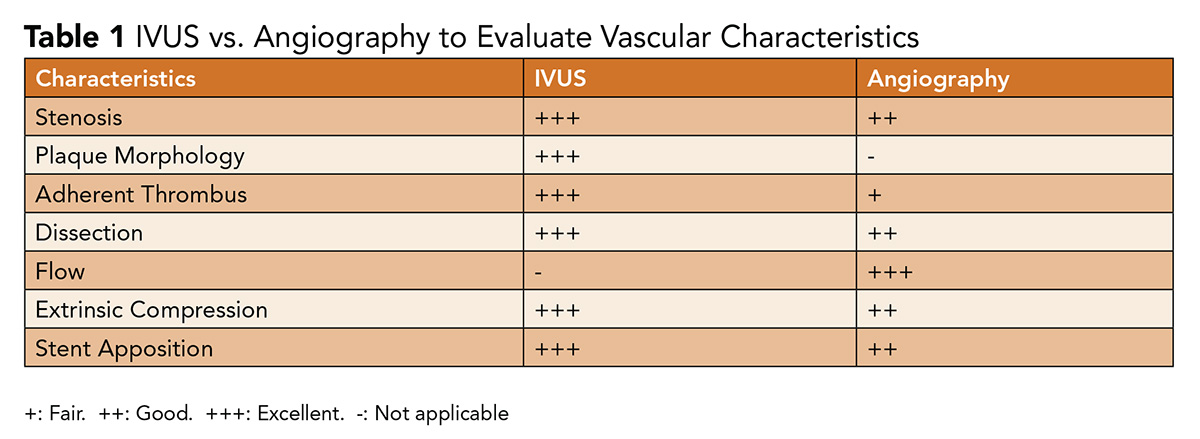
Angiography produces a 2-dimensional silhouette of a 3-dimensional vessel, with findings and lesion characteristics highly dependent on projection. This can make angiography challenging to interpret in the setting of vascular tortuosity and it lacks the ability to display the presence of eccentric plaque; delineate plaque characteristics; reliably assess for adherent thrombus; or guide stent sizing and apposition (Table 1).
These data are all critical in ensuring patency and improving outcomes.1
As IVUS is able to provide an intraluminal view of the vessel, it can fill many of these gaps, and can be combined with invasive hemodynamics and other imaging modalities, such as computed tomography (CT), to provide a detailed roadmap for vascular interventions.
Emerging data have supported its use as an adjunct to traditional angiography.
IVUS-Guided Venous Intervention
The use of IVUS for venous imaging and intervention is quickly becoming adopted as a standard of care due to the advantages it presents compared with venography alone. IVUS has the capacity to visualize nonocclusive thrombi that may be missed on traditional venography and aid in distinguishing chronic vs. acute thrombosis.2
It can define luminal features, such as trabeculations and valvular issues, that cannot be captured on venography, as contrast obscures the lumen.3
Furthermore, veins are particularly susceptible to extrinsic compression from other vascular and anatomical structures, which can be more accurately assessed by intraluminal imaging compared with venography that only produces 2-dimensional planar views.3
The use of IVUS to examine the degree and length of venous stenosis, in combination with information from other imaging modalities, such as CT, duplex ultrasound and venous pressure transduction, can be crucial to planning PVI.
In ilio-caval interventions, the use of IVUS can identify landing zones of healthy vessel walls to bypass long segments of venous disease that may be underappreciated with venography.4 The increased accuracy in luminal stenosis can also aid in determining stent sizing, which can reduce the risk of thrombosis and poor long-term patency.5
Outcomes in Venous Interventions

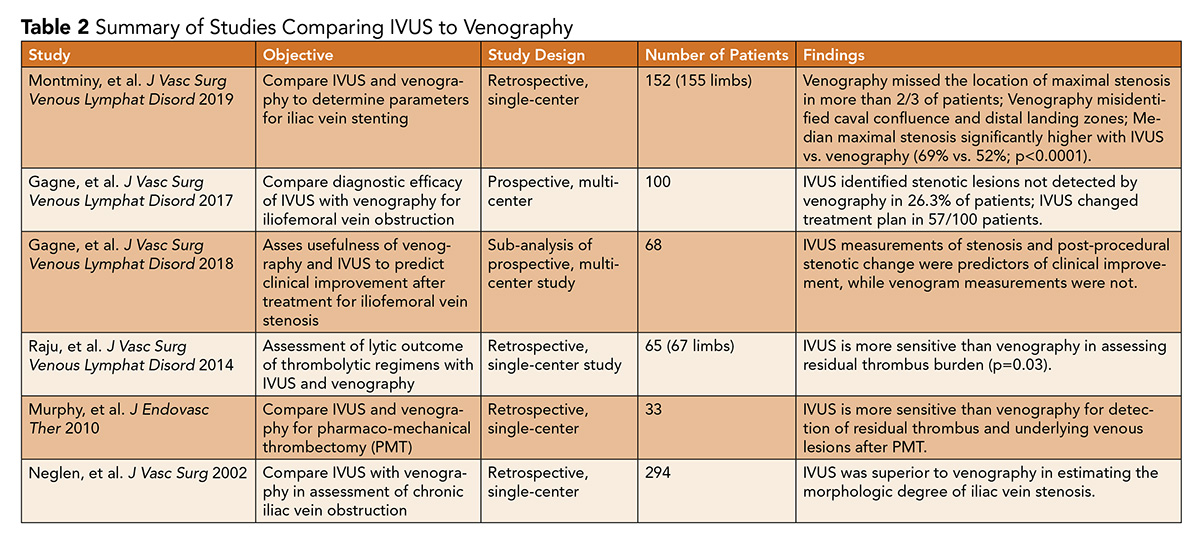
There are strong data supporting the use of IVUS as an adjunct for the treatment of venous stenosis, extrinsic venous compression, deep vein thrombosis, and post-thrombotic syndromes. The Venogram vs. IVUS for Diagnosing Iliac Vein Obstruction (VIDIO) trial was a single-arm, prospective, multicenter study (n=100) that evaluated the merits of venography vs. IVUS in diagnosing common iliac and common femoral vein stenosis.
The study demonstrated that IVUS identified significant lesions in 26.3% of patients not initially detected by venography and resulted in a change in treatment plan for 57/100 patients.6 These data clearly illustrate what IVUS provides – increased vessel definition when assessing for treatable iliofemoral vein stenoses.
Montminy, et al., presented a retrospective study of 155 limbs with chronic iliac vein occlusion treated with IVUS-assisted stenting between 2013 and 2015.
The study found that venography was inferior to IVUS in delineating the degree of stenosis, location of stenosis and optimal proximal and distal landing zones for venous stents.4 This is of particular significance as inappropriate identification of landing zones and stent malposition can lead to a need for reintervention.7
In addition to aiding procedural success, there are data to suggest that IVUS may have a predictive role in determining symptom response to interventions. Gagne, et al., presented data from the VIDIO trial that demonstrated IVUS-derived measurements of baseline stenosis and stenotic change after intervention had significant utility in predicting postprocedural clinical improvement in symptoms, while venographic measurements did not.8
IVUS use in deep vein thrombosis (DVT) intervention has demonstrated a role in identifying residual thrombus burden after mechanical thrombectomy. A prospective single center study of 33 patients undergoing percutaneous thrombectomy with venography and IVUS pre- and post-intervention was notable because IVUS was able to identify residual thrombus, stenosis and May-Thurner anatomy requiring further intervention, all of which were unidentified by venography.9
Raju, et al., presented a cohort of patients undergoing percutaneous thrombectomy for DVT (35 limbs) or iliac vein stent thrombosis (32 limbs), demonstrating that residual thrombus detected by IVUS was present in 91% of treated limbs.10
Some centers have shown success with using IVUS as the primary imaging modality in populations that are vulnerable to contrast or radiation. For instance, Alhalbouni, et al., presented data from one treatment center that progressed to utilizing IVUS alone without angiography in patients with renal insufficiency, eliminating the need for contrast guidance in the treatment of venous outflow obstruction.11
Use of IVUS to guide thrombectomy in a pregnant patient with phlegmasia cerulea dolens, sparing both contrast and radiation, has also been described.12
Despite the strength of IVUS as an adjunctive tool in venous interventions, and potentially a primary imaging modality in certain high-risk populations in the future, large-scale randomized trials with head-to-head comparisons of IVUS-assisted and IVUS-unassisted venous interventions are limited (Table 2).
Furthermore, the lack of procedural standardization in PVI makes it challenging to compare different techniques, as operator variability may diminish the power to detect differences in efficacy when comparing IVUS-assisted procedures to IVUS-unassisted procedures.
Despite the recognized strengths of IVUS in venous intervention, consensus guidelines and appropriate use criteria are lacking.13,14
IVUS-Guided Arterial Intervention
In the sphere of arterial interventions, IVUS provides several practical advantages compared with angiography alone. IVUS has the capacity to define atherosclerotic plaque morphology, length and eccentricity with high precision.
With image processing tools that translate echogenicity into profiles for different tissues, IVUS can subclassify plaques into four major categories: 1) fibrous, 2) fibro-fatty, 3) necrotic-lipid and 4) calcific.15 This is of particular significance as there is evidence to suggest that calcification in peripheral arteries is more accurately assessed by IVUS than angiography alone.16 Furthermore, it can gauge the degree of stenosis more accurately compared with angiography.17,18
These factors can play an integral role in guiding adequate preparation of the vessel using techniques such as atherectomy, which has been shown to improve the success of peripheral intervention involving highly calcified plaques.19
Deployed stents can subsequently be examined for compression, inadequate expansion and apposition that may not be seen on angiography, despite an apparently adequate angiographic result.20,21 IVUS has also been shown to be more sensitive at detecting postprocedural complication such as vascular dissections.22
Outcomes in Arterial Interventions

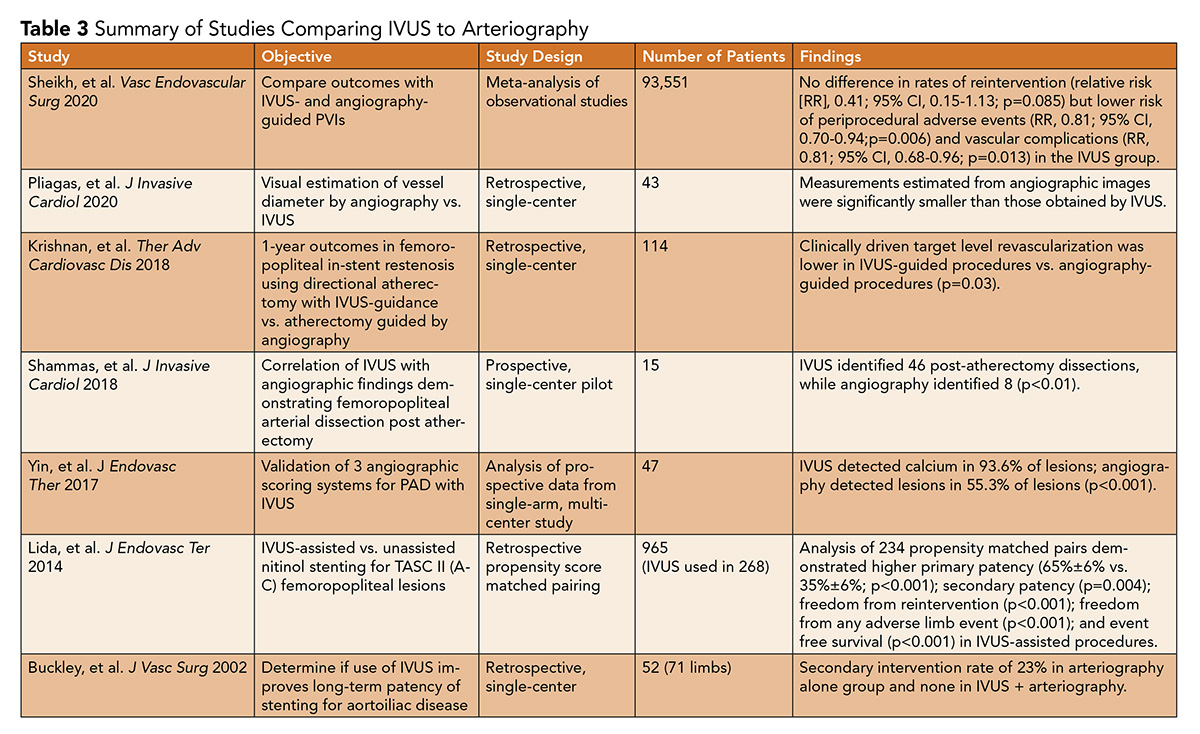
Despite the mechanistic utility of better plaque characterization, guidance of vessel preparation, and benefits regarding improved assessments of stent sizing and apposition, the data for use of IVUS in arterial interventions in patients with PAD are limited. Kamakura, et al., published a retrospective cohort study of 15-year patency rates among patients with PAD undergoing endovascular therapy with stenting guided by IVUS.
Among 455 patients (mean age, 72 years), in whom endovascular therapy was performed on 507 lesions, the 5-, 10- and 15-year primary patency rates were 89%, 83% and 75%, respectively.
Interestingly, the rates of patency were not different among patients with different TransAtlantic Inter-Society Consensus (TASC) II lesion complexity (A through D; D, highest severity). There was also a high success rate with primary stenting (97.2%).23
Several limitations of this study included the low rate of critical limb ischemia in the overall cohort as well as high attrition rates due to mortality, which may introduce bias magnifying the benefit of IVUS-guided endovascular therapy.24
Another challenge in understanding the benefit of IVUS-guided therapy in PVI relates to the lack of a reliable comparator arm, particularly that of angiography alone. Studies that utilize IVUS are also retrospective and have relatively small sample sizes.
A cohort study by Buckley, et al., of 52 patients (36 with IVUS-assisted intervention) undergoing balloon angioplasty and stenting of aortoiliac disease showed that procedures that did not use IVUS had a restenosis rate of 18%, while those with IVUS assistance had none.25
A retrospective study by Krishnan, et al., of 114 patients reported lower clinically driven target level revascularization related to femoropopliteal in-stent restenosis when comparing IVUS-assisted atherectomy to angiography-assisted atherectomy.19
However, larger metanalyses have shown mixed results with regard to freedom from reintervention.26,27
Notably, there are no comparative data examining the benefit of IVUS-guided therapy in infrainguinal disease.
A practical challenge related to the use of IVUS in peripheral artery interventions is the lack of consensus guidelines detailing appropriateness of use. Thus far, studies detailing cost-effectiveness are also limited.
A study by Panaich, et al., of 92,714 patients from the Nationwide Inpatient Sample database revealed a nonsignificant increase in hospital costs ($1,333; 95% confidence interval [CI) – $167 to +$2,833; p<0.001), but a significant reduction in postprocedural complications (odds ratio, 0.80; 95% CI, 0.66 to 0.99, p=0.037).28
Given the lack of consensus recommendations and minimal prospective trial data examining arterial interventions (Table 3), reimbursement from health care payers has been limited. Additional prospective and well-powered studies are certainly warranted to evaluate IVUS-assisted interventions compared with angiography alone.
Conclusions
IVUS provides a valuable intra-luminal perspective for both arterial and venous interventions. This ranges from clearly defining intravascular lesions to guiding appropriate stent sizing and placement, with the goal of better optimizing procedural outcomes.
Although the data supporting use in venous intervention is greater, IVUS is not currently included in the society guidelines and adoption rates are unclear. There is now a pressing need to re-evaluate the importance of IVUS as an adjunct for peripheral interventions, identify gaps in data, and consider updating consensus guidelines and appropriate use criteria.
References
- Lee JT, Fang TD, White RA. Applications of intravascular ultrasound in the treatment of peripheral occlusive disease. Semin Vasc Surg 2008;19:139-44.
- McLafferty RB. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol 2012;29:10-15.
- Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002;35:694-700.
- Montminy ML, Thomasson J, Tanaka G, et al. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord 2019; 7:801–807.
- Murphy EH, Blake J, Varney E, et al. Deep venous thrombosis associated with caval extension of iliac stents. J Vasc Surg Venous Lymphat Disord 2017;5:8-17.
- Gagne PJ, Tahara R, Fastabend C, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678-687.
- Raju S, Tackett P, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg 2009;49:511-8.
- Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018;6:48-56.e1.
- Murphy EH, Broker H, Johnson E, et al. Device and imaging-specific volumetric analysis of clot lysis after percutaneous mechanical thrombectomy for iliofemoral DVT. J Endovasc Ther 2010;17:423-33.
- Raju S, Martin A, Davis M. The importance of ivus assessment in venous thrombolytic regimens. J Vasc Surg Venous Lymphat Disord 2013;1:108.
- Alhalbouni S, Hingorani A, Shiferson A, et al. Iliac-femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg 2012;26:185-9.
- Dua A, Rothenberg KA, Rao C, et al. Thrombolysis for management of phlegmasia cerulea dolens in the first trimester of pregnancy. Ann Vasc Surg 2019;59:313.e1-313.e3.
- Lee DW, Cavender MA. Guidelines for peripheral vascular disease: where is the evidence? Circ Cardiovasc Interv 2019;12:e007561.
- Sardar P, Giri J, Jaff M, et al. Strength of evidence underlying the American Heart Association/American College of Cardiology guidelines on endovascular and surgical treatment of peripheral vascular disease. Circ Cardiovasc Interv 2019;12;e007244.
- Suh WM, Seto AH, Margey R JP, et al. Intravascular detection of the vulnerable plaque. Circ: Cardiovasc Imaging 2011;4:169-178.
- Yin D, Maehara A, Shimshak T, et al. Intravascular ultrasound validation of contemporary angiographic scores evaluating the severity of calcification in peripheral arteries. J Endovasc Ther 2017;24:478-87.
- Arthurs ZM, Bishop P, Feiten LE, et al. Evaluation of peripheral atherosclerosis: a comparative analysis of angiography and intravascular ultrasound imaging. J Vasc Surg 2010;51:933-8.
- Pliagas G, Saab F, Stavroulakis K, et al. Intravascular ultrasound imaging versus digital subtraction angiography in patients with peripheral vascular disease. J Invasive Cardiol 2020;32:99-103.
- Krishnan P, Tarricone A, K-Raman P, et al. Intravascular ultrasound guided directional atherectomy versus directional atherectomy guided by angiography for the treatment of femoropopliteal in-stent restenosis. Ther Adv Cardiovasc Dis 2018;12:17-22.
- Rosenfield K, Schainfeld R, Pieczek A. Restenosis of endovascular stents from stent compression. J Am Coll Cardiol 1997;29:328-38.
- Navarro F, Sullivan TM, Bacharach JM. Intravascular ultrasound assessment of iliac stent procedures. J Endovasc Ther 2000;7:315-9.
- Shammas NW, Torey JT, Shammas WJ, et al. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: results from the iDissection study. J Invasive Cardiol 2018;30:240-4.
- Kumakura H, Kanai H, Araki Y, et al. 15-year patency and life expectancy after primary stenting guided by intravascular ultrasound for iliac artery lesions in peripheral arterial disease. JACC Cardiovasc Interv 2015;8:1893-1901.
- Shishehbor MH, Agarwal S. Imaging-guided lower extremity endovascular interventions: is now the time? JACC Cardiovasc Interv 2015;8:1902-4.
- Buckley CJ, Arko F, Lee S, et al. Intravascular ultrasound scanning improves long-term patency of iliac lesions treated with balloon angioplasty and primary stenting. J Vasc Surg 2002;35:316-23.
- Sheikh AB, Anantha-Narayanan M, Smolderen K, et al. Utility of intravascular ultrasound in peripheral vascular interventions: systematic review and meta-analysis. Vasc Endovascular Surg 2020;54:413-22.
- Lida O, Takahara M, Soga Y, et al. Efficacy of intravascular ultrasound in femoropopliteal stenting for peripheral artery disease with TASC II class A to C lesions. J Endovasc Ther 2014;21:485-92.
- Panaich SS, Arora S, Patel N, et al. Intravascular ultrasound in lower extremity peripheral vascular interventions: variation in utilization and impact on in-hospital outcomes from the Nationwide Inpatient Sample (2006-2011). J Endovasc Ther 2016;23:65-75.
Clinical Topics: Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Stable Ischemic Heart Disease, Vascular Medicine, Aortic Surgery, Cardiac Surgery and Heart Failure, Cardiac Surgery and SIHD, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging, Chronic Angina
Keywords: ACC Publications, Cardiology Magazine, Pregnancy, Prospective Studies, Phlebography, Constriction, Pathologic, Thrombectomy, Venous Thrombosis, Thrombosis, Angiography, Iliac Vein, Renal Insufficiency, Cohort Studies, Stents, Phlebography, Constriction, Pathologic, Femoral Vein, Retrospective Studies, Postthrombotic Syndrome, Standard of Care, Tomography, X-Ray Computed, Venous Pressure, Hemodynamics, Odds Ratio, Ultrasonography, Interventional, Confidence Intervals, Cost-Benefit Analysis, Inpatients, Coronary Restenosis, Hospital Costs, Atherectomy, Goals, Angioplasty, Balloon, Arteries
< Back to Listings