Cover Story | ECMO: Lessons Learned From the COVID Era
A 31-year-old woman with COVID-19 infection was admitted on October 26, 2021, to University Hospital Split (Croatia) with shortness of breath, cough and weakness. She was 33 weeks pregnant and had tested positive for SARS-CoV-2 six days earlier.
"Given that the gestational age of the child was quite advanced, and the mother's health was deteriorating quickly, we decided to do a C-section," said anesthesiologist Filip Peris, MD, in a June press release from the Euroanaesthesia Congress in Milan, Italy. The baby, a boy weighing 5 lb. 4 oz., was healthy and did not require any special medical care.
The mother, who had not been vaccinated against COVID-19 and had no significant underlying medical conditions, was transferred to the hospital's COVID ICU and placed on a ventilator. Her lung function ratio (P/F) was 85. (Ratios below 100 are classed as severe acute respiratory distress syndrome or ARDS). Her lung function ratio further deteriorated to 70 and she was placed on extracorporeal membrane oxygenation (ECMO) to allow her lungs to heal.
"ECMO was her last chance," said Peris. The patient's lung function gradually improved and she was weaned off ECMO after nine days. After 22 days in hospital, she was discharged home and has since made a full recovery.
"Our hospital delivered more babies in the cardiovascular intensive care unit (CVICU) during COVID than I've seen throughout my entire career, because we were supporting the mothers on ECMO to get their babies to viability," says Shelley A. Hall, MD, FACC. "Despite that, we still lost some babies because they couldn't oxygenate well enough," she adds.
Hall is chief of Transplant Cardiology, Heart Failure and Mechanical Support at Baylor University Medical Center (Dallas, TX). She also serves as chair of the UNOS Cardiac Committee and is president and governor of ACC's Texas Chapter.
ECMO Offers a "Last Chance"

One of the things you always want to think about when considering putting somebody on ECMO is where are you going? Because you want to make sure if recovery happens, the body has tolerated the intervention.
– Shelley A. Hall, MD, FACC
In early 2020, physicians in Wuhan, China and Italy started using ECMO to support the very sickest patients with COVID-19 illness when high-flow oxygen and mechanical ventilation was not sufficient. By the time North America was shutting down in March, they were already publishing their findings.1,2
"It was horrible to see how this virus just turned lung tissue to pulp. But being on 100% O2 for prolonged periods also destroys the lungs. We'd try to put some of these patients on VV-ECMO, but we saw a lot of patients who were unrecoverable – they needed a lung transplant to survive," says Jaime D. Moriguchi, MD, FACC, director of the Mechanical Circulatory Support Program Advanced Heart Disease at Cedars-Sinai Medical Center (Los Angeles, CA).
Adds Hall, "All of a sudden, COVID was everywhere and people were dying. We had to quickly figure out all the ways in which we needed to do ECMO better to fit the situation."
Physicians were also forced to work out how to identify people most likely to benefit from the resource-heavy strategy, something driven home when they realized that, with COVID, patients might need to stay on ECMO for weeks or months, increasing the likelihood that an allocated machine would remain allocated for a long time.
"We lost many, many people because there was simply no way to keep up with the demand," says Hall. At one point, in her hospital system, there were 70 patients on the wait list for ECMO.
"It was a mass casualty triage, sometimes on an hourly basis," says Hall. "If a bed or equipment opened up, either from recovery or death, first thing you did was look at the list and try to figure out who's got the best chance of survival."
The problem wasn't always just about having an available machine. Staffing was an issue too. "Nurses were leaving to be travel nurses because the companies were offering three times the salary," says Hall. "We often had more machines but were never able to run them all at one time because of staffing."
As a busy transplant center that did not stop transplants during COVID, they also had to keep ECMO beds available for cardiac patients. One ECMO specialist can manage two to four beds, but only with each bed also having a dedicated nurse, notes Hall.
Patient selection was made harder in Texas when the governor made it illegal to consider vaccination in the allocation of medical resources for COVID-19. "Unvaccinated patients were much less likely to survive severe COVID, but no Texas hospital could formally consider vaccination status of the patient. The political environment interfered with medical decision-making at times."
"Normally, before COVID, we might have three or four beds in our 24-bed cardiac surgery ICU taken up by ECMO patients. During the height of COVID, in January 2021, we had 14 of our ECMO units in use at one time," says Moriguchi.
"During our second major COVID surge last January, we had eight patients on VV-ECMO with an average age of 34 years, no identifiable comorbidities and the only unifying factor was that they were all unvaccinated. This obviously brings into play a great number of ethical issues," he adds.
Evolution Spurred By the Virtual Grapevine
Much of what North American clinicians needed to know about ECMO for COVID was passed along informally through personal networks, evolving organically, says Hall. "But also, volume always drives efficiency and we most certainly had volume," she adds.
Most of what changed in how ECMO was delivered were efficiencies related specifically to COVID (e.g., proning) or to improve safety and longevity on the device. These included how to transport a patient on ECMO, how to cannulate someone in the field and take them to the hospital center, cannula stabilization techniques and improved anticoagulation.3
"Early on we'd be in touch with a colleague in a hot spot and hear about their experiences. Then people started forming big WhatsApp groups around the world where we would discuss best practices and people would share what they discovered works with others," says Hall. "Ultimately, we started doing weekly virtual meetings on Webex."
As the virtual world took over, collaboration increased and what had previously been a silo for the transplant space and surgery, "became this massive collaboration between critical care medicine, advanced heart failure cardiology and thoracic surgery, so there was this massive team approach to it all," says Hall.
The Extracorporeal Life Support Organization (ELSO), a nonprofit organization that supports health care professionals and scientists involved in ECMO, also played a part. They facilitated real-time sharing of clinical experiences through discussion boards and webinars and brought together teams of international experts to publish a guidance document.
In 2021, they published COVID-19 ECMO guidelines to represent the accumulated global knowledge on subjects such as resource utilization, cannulation technique, anticoagulation use and personal protective equipment use.4 And as the field's repository of data, ELSO plays an important role in tracking outcomes.
Making it Last

As an example, we'll put a heart transplant patient with refractory rejection on VA-ECMO because often we can turn it around if we can support them long enough to give the drugs a chance to work.
– Jaime D. Moriguchi, MD, FACC
ECMO is a treatment normally indicated to provide respiratory and circulatory support for a period of days or weeks and not for months (except in children who are able to tolerate the treatment for longer periods). That changed during COVID.
"Before COVID, we'd placed patients on ECMO for temporary support, but suddenly we had many patients on VV-ECMO for months, which was extremely draining on resources and staff morale," says Moriguchi.
Longer durations of support are only possible with VV-ECMO, he adds. In adults, with VA-ECMO, "complications usually occur by the second or third week. If you have a patient who lasts four weeks on VA-ECMO, it would be extremely unusual."
It should be noted that, in the ELSO COVID registry, only 4% of patients received VA-ECMO. "We had many patients on VV-ECMO whose hearts started to fail and most of them were not switched to VA-ECMO. It's basically just a question of switching up the cannula from venous to arterial access, but if the heart failed, the chance of survival was very low," says Hall.
Advanced age is a potential but not absolute contraindication to ECMO.4 "With COVID, many of the patients who stayed on ECMO for months were young, so their kidneys and other organs were young and healthy and they could survive. Otherwise, most patients will ultimately develop multisystem organ failure, infection, bleeding or stroke if they're on ECMO for more than a few weeks, even VV-ECMO, but especially VA-ECMO," says Moriguchi.
New ACC/AHA Data Standards Address CV, Non-CV Complications of COVID-19

The ACC and the American Heart Association (AHA) Task Force on Clinical Data Standards have published a new document listing and defining key data elements related to cardiovascular and noncardiovascular complications of COVID-19. The data elements are meant to be applicable to the full range of care provided to patients with confirmed, probable and suspected acute COVID-19, as well as those with postacute sequelae, and are intended for use across ambulatory and hospital settings.
The new report also defines acute cardiovascular complications related to COVID-19, including acute myocardial injury, heart failure, shock, arrhythmia, thromboembolic complications and stroke, and provides data elements related to COVID-19 vaccination status, comorbidities and pre-existing cardiovascular conditions. Additionally, data elements for cardiovascular mortality during acute COVID-19, and noncardiovascular complications (which can provide a fuller picture of the severity of illness) are also provided.
Other key highlights from the document include a listing of COVID-19 signs and symptoms, as well as defined data elements for diagnostic and therapeutic strategies. Advanced therapies such as mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and end-of-life management are also addressed.
"These data standards will help standardize definitions and set the framework to capture and better understand how COVID-19 impacts cardiovascular health," write Biykem Bozkurt, MD, PhD, FACC, FAHA, et al. "This document is intended for use by researchers, registry developers, and clinicians and is proposed as a framework for ICD-10 code development of COVID-19-related cardiovascular conditions."
The report was endorsed by the Heart Failure Society of America and the Society for Cardiac Angiography and Interventions. Click here for the full document published in JACC.
This made it even more crucial to better manage the usual complications of ECMO – namely bleeding, infection, stroke, clot formation and lower limb ischemia (due to occlusion of arterial inflow).
"One of the things you always want to think about when considering putting somebody on ECMO is where are you going? Because you want to make sure if recovery happens, the body has tolerated the intervention," says Hall.
Particularly with a longer duration, immobilization becomes a major issue. "Patients develop critical polyneuropathy, lose muscle mass and strength, and develop bed sores and deep vein thrombosis. All of these things impact their ability to come back," she says. "We had patients in slings and on chairs and we were doing everything we could to get them mobile to promote that aspect of recovery."
As with other high-complexity interventions, ECMO outcomes vary across centers. And volume matters. The real-time, global ELSO COVID-19 registry puts in-hospital mortality at 47% in the 13,493 reported cases (as of June 28, 2022) who initiated ECMO at least 90 days before. (Note: not all ECMO centers are part of ELSO or reported outcomes to their registry.)
In other studies, the cumulative incidence of in-hospital mortality 90 days after ECMO initiation has ranged from 31% to 59%.4,5 Adams, et al., reported their single-center outcomes from Advocate Christ Medical Center in Oak Lawn, IL, a high-volume referral center, at ACC.21. From 40 patients (mean age, 46.8 years; 73% male) treated with VV-ECMO, they found that 73% of their patients survived until hospital discharge. The median duration of ECMO support was 25 days.
Moriguchi and Hall both say survival at their centers exceeded 70% for VV-ECMO. "Our survival improved over time, so I think all the efficiencies paid off," says Hall.
For Moriguchi, as bad as the COVID pandemic was, a great deal was learned from managing those critically ill patients. "It is important to keep in mind that ECMO does not treat COVID. It just supports them until we can either treat them with specific therapy or the body fights off the virus." He notes that by keeping people alive, much was learned about how this particular virus stimulates a cascade of different biochemical events in the human body, causing a tremendous inflammatory response that affects the lungs and causes clotting, pulmonary emboli, clotting in the coronary arteries and direct myocarditis.
"It's been tough, but I think we're a lot smarter about it now, and for most people we can turn around the virus and they won't die of COVID," he adds.
Overall, there are no data to recommend deviation from conventional ECMO management for COVID-19 patients during their ECMO run, according to current guidelines.4
"It's always hard to say there's a silver lining to COVID, but the efficiencies we developed during COVID made a difference in outcomes and will continue to improve ECMO outcomes going forward. I would suggest it advanced ECMO science by about a decade in a matter of months," says Hall.
This article was authored by Debra L. Beck, MSc.
References
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-69.
- Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med 2020;8:240-1.
- Dalia AA, Convissar D, Crowley J, et al. The role of extracorporeal membrane oxygenation in COVID-19. J Cardiothorac Vasc Anesth 2022;May 10:[Epub ahead of print.]
- Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J 2021;67:485-95.
- Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021;398:1230-38.
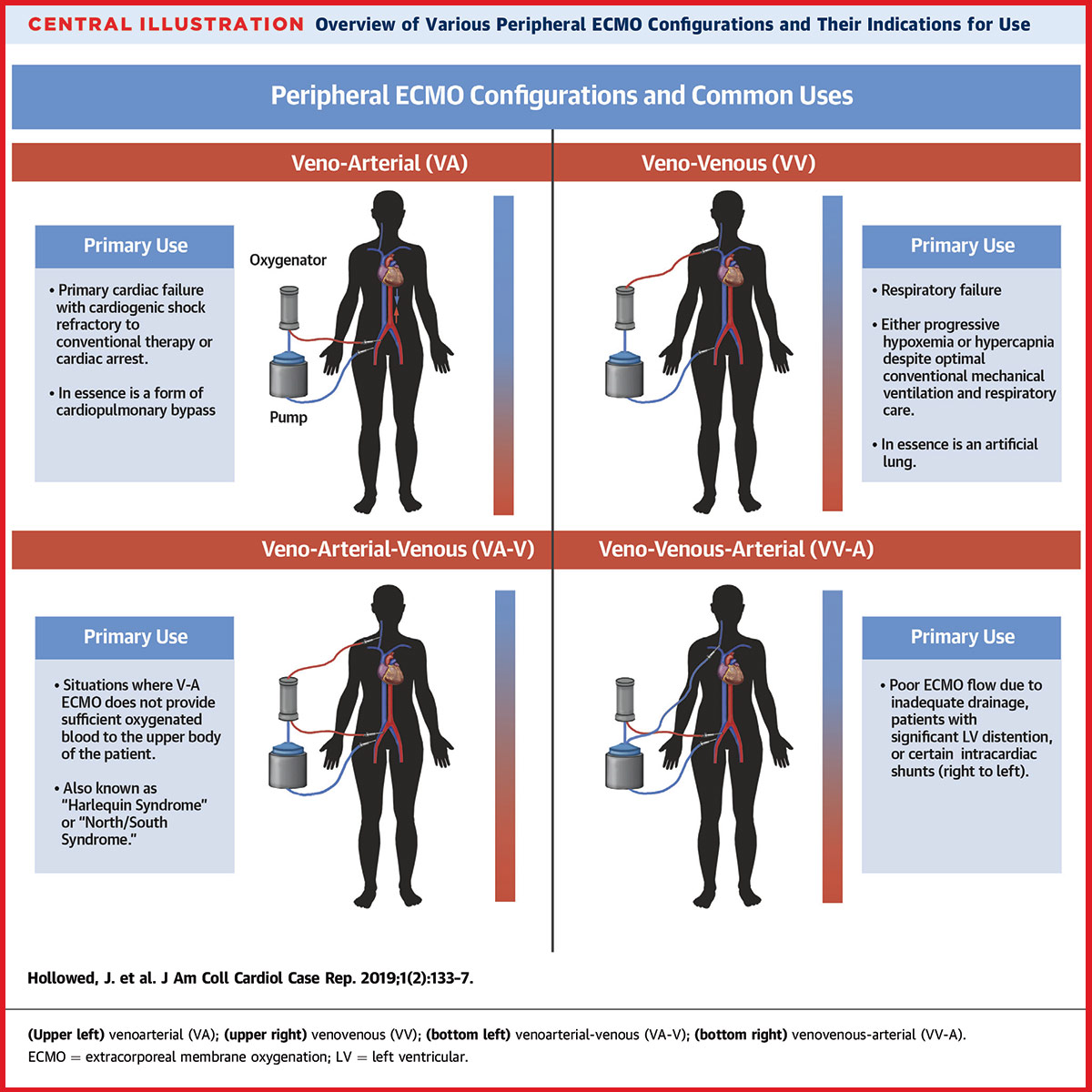
ECMO Basics
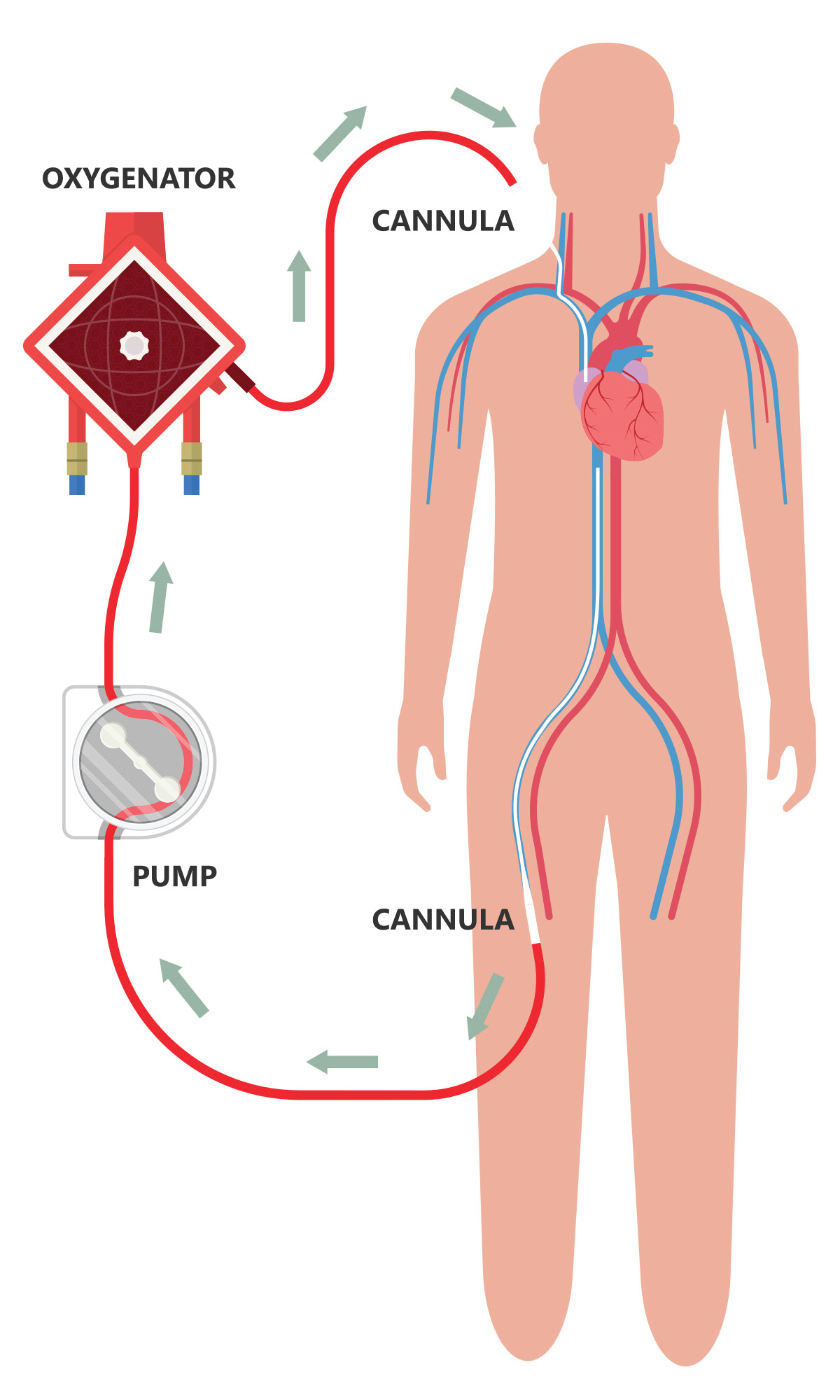
ECMO is the most aggressive form of life support available. It can be configured in two ways (Figure). Venovenous (VV) ECMO primarily provides respiratory support to a patient whose heart is still functioning well enough to meet the body's needs. In VV-ECMO, deoxygenated blood is taken from a large vein, scrubbed of CO2 and reoxygenated, and then returned to the venous circulation to be circulated by the heart.
VV-ECMO is used to provide oxygenation and CO2 removal, or both, in the case of reversible respiratory failure, including ARDS, either due to bronchopulmonary aspiration; bacterial, viral or atypical pneumonia; barotrauma; or acute or chronic interstitial pneumonitis. It is also used as a bridge to transplant in case of end-stage lung disease.1
Venoarterial (VA) ECMO, on the other hand, functions much like cardiopulmonary bypass and supports both the heart and lungs. During VA-ECMO, deoxygenated blood is withdrawn from the venous system and oxygenated blood is returned to the arterial system, allowing oxygen-rich blood to circulate through the body even if the heart is too weak to pump it.
Both VV- and VA-ECMO are usually employed in the cardiac ICU, with cardiothoracic surgeons (among others) charged with placing the cannulae and perfusionists or ICU nurses charged with 24-hour monitoring. The machinery is the same, just with different cannula placement.
The indications for VA-ECMO fall broadly into four categories:2
- Cardiogenic shock refractory to conventional treatments, including an existing mechanical circulatory support device such as an intra-aortic balloon pump
- Witnessed cardiac arrest in those who do not regain native circulation with conventional cardiopulmonary resuscitation
- Refractory ventricular arrhythmias
- Acute or decompensated right heart failure in the context of pulmonary vascular disease (e.g., pulmonary hypertension or pulmonary embolism)
"As an example, we'll put a heart transplant patient with refractory rejection on VA-ECMO because often we can turn it around if we can support them long enough to give the drugs a chance to work," says Moriguchi.
"Honestly, though, we try to avoid using ECMO as much as possible," he adds. Before a decision is made to use ECMO, advanced heart failure specialists (and the intensivists, cardiothoracic surgeons and hospitalists who work along with them) will first try any of a number of temporary mechanical circulatory devices available to support cardiac function, such as Impella, a tiny pump inside a catheter that can provide up to 5 liters per minute of forward flow from the left ventricle to the aorta.
"Before ECMO, the surgeons will place an Impella catheter in the hope that by supporting the left side, it'll be possible to prevent the right side of the heart from dying and to preserve the organ," says Moriguchi.
Also, says Hall, "VA-ECMO is bad for the heart. It pulls blood out of the body from the right side of the heart, usually the right atrium or the vena cava, oxygenates it and then delivers it back into, usually, the femoral artery or into the upper arterial system." This increases the afterload on the heart, she explains, making it work harder to eject against a higher pressure, and working against the fact that the patient is on VA-ECMO because the heart isn't working. This sometimes weakens the heart further such that a second device is needed to "vent" the heart by pulling blood directly out of the left side to counteract rising pressures.
References
- Makdisi G, Wang I-wen. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-E176.
- Bermudez CA, Daneshmand ME. Chapter 6: Role of Extracorporeal Membrane Oxygenation. In: Kirklin James K, Rogers JG, eds. Cardiac Support in Mechanical Circulatory Support: A Companion to Braunwald's Heart Disease 2nd Ed. Philadelphia, PA. 2020: Pg. 59.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, COVID-19 Hub, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Prevention, Vascular Medicine, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Interventions, CHD and Pediatrics and Quality Improvement, Acute Heart Failure, Interventions and Vascular Medicine
Keywords: ACC Publications, Cardiology Magazine, Academic Medical Centers, Anesthesiologists, Anticoagulants, Cardiac Surgical Procedures, Catheterization, Clinical Decision-Making, Coronary Vessels, Contraindications, Cough, COVID-19, Critical Care, Critical Illness, Dyspnea, Extracorporeal Membrane Oxygenation, Gestational Age, Heart Diseases, Health Personnel, Hospital Mortality, Hospitals, Heart Failure, Human Body, Intensive Care Units, Infant, Newborn, Ischemia, Kidney, Longevity, Lung Transplantation, Lung, Mass Casualty Incidents, Muscles, Myocarditis, Organizations, Nonprofit, Pandemics, Oxygen, Patient Discharge, Patient Reported Outcome Measures, Patient Selection, Personal Protective Equipment, Polyneuropathies, Pregnancy, Pressure Ulcer, Referral and Consultation, Registries, Respiratory Distress Syndrome, Salaries and Fringe Benefits, SARS-CoV-2, Stroke, Thoracic Surgery, Triage, Vaccination, Venous Thrombosis, Ventilators, Mechanical, Workforce
< Back to Listings