Peripheral Matters | Current Status of Interventional Therapies in Acute Pulmonary Embolism
Pulmonary embolism (PE) remains the third leading cause of cardiovascular death. Although major advances in care have been made for myocardial infarction and stroke, PE has yet to experience its clinical renaissance.1
We and others recently published an American Heart Association Scientific Statement on interventional therapies for acute PE to consolidate and summarize current knowledge regarding existing and new therapies emerging in the field of PE management.2
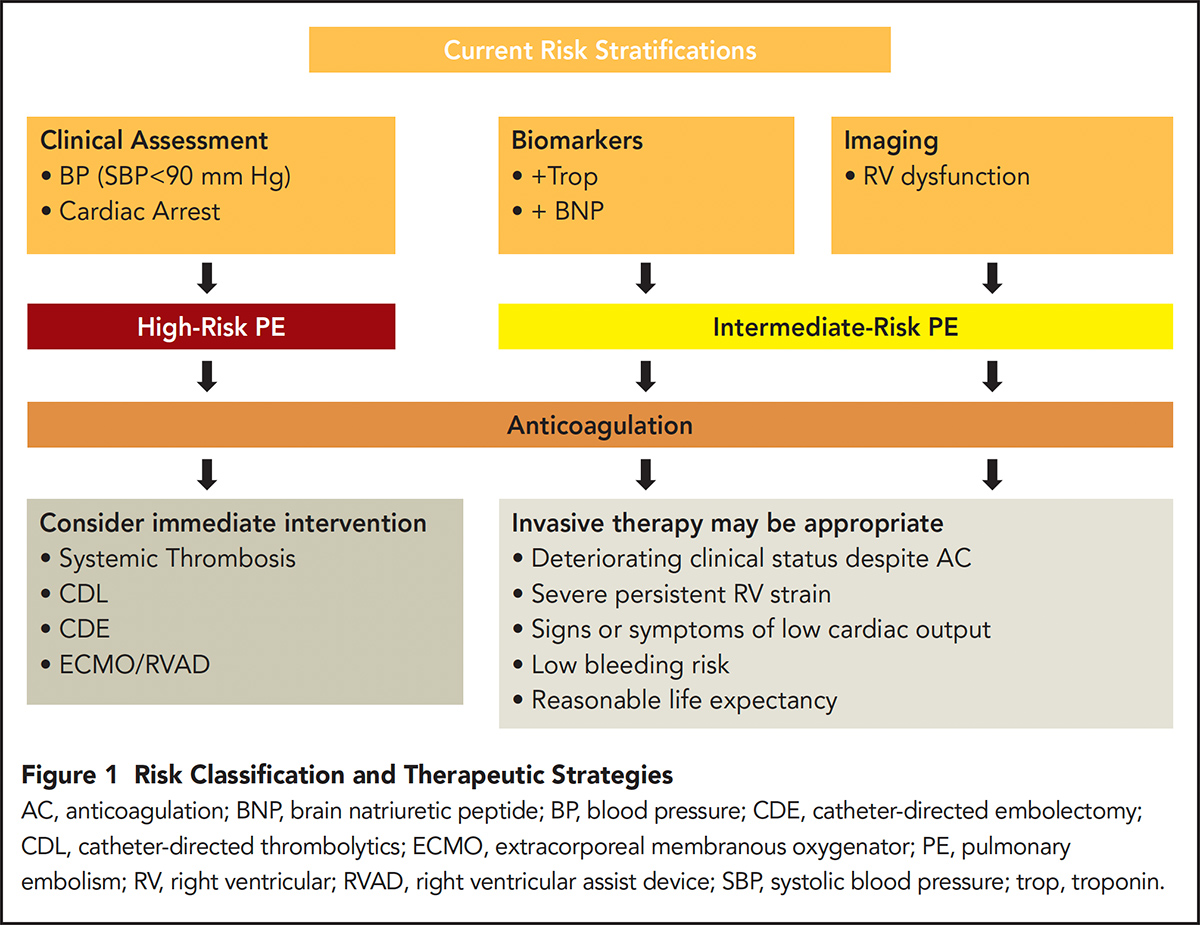
PE severity is dynamic, ranging from asymptomatic to life threatening. An individual patient may slide in either direction along this continuum at any given point during their course. While current risk stratification models attempt to group patients based on acute hemodynamic status, the individual risk of mortality is difficult to predict as half of observed deaths are from non-PE related causes.
However, current risk stratification models appear to correlate with short-term mortality. Three categories of PE risk are recognized by the scientific statement: low, intermediate and high.3

High-risk PE is defined by hypotension with systolic blood pressures <90 mm Hg or need for vasopressor support. These patients compromise a minority of cases (about 5 percent of all hospitalized patients) but carry the worst prognosis with mortality rates of approximately 30 percent within one month.4-6 Intermediate-risk PE is defined by right ventricular (RV) strain but without hypotension.
RV strain can be identified by computed tomography (CT) pulmonary angiography or echocardiography with RV/left ventricular (LV) ratios of >0.9, or biomarkers consistent with RV injury including elevated troponins or brain natriuretic hormone.
The intermediate category of PE risk applies to roughly a third to half of hospitalized PE patients. Mortality in this group ranges from 2-15 percent at 30 days.7-10 Finally, low-risk PE is that which does not meet any of the aforementioned criteria and account for the majority of all PE diagnoses.
These patients have an average mortality of 1 percent within the first month of diagnosis.11
Management of PE

The upfront management of PE is guided by the initial presentation. For patients who present with low-risk PE, anticoagulation alone is recommended. Anticoagulation is the mainstay for the treatment of all PE regardless of risk.
More aggressive options may be considered for patients presenting with intermediate- and high-risk PE. In general, these options can be grouped into three different categories: systemic thrombolysis, catheter-directed interventions and surgical embolectomy.
For systemic thrombolysis, the largest trial performed to date is the PEITHO trial in which 1,006 patients presenting with intermediate-risk PE were randomized to receive systemic tenecteplase vs. anticoagulation alone.12
In this study, administration of systemic thrombolysis demonstrated benefit for the combined endpoint of mortality or hemodynamic collapse, driven mostly by the latter. However, this came at the cost of increased major bleeding, specifically intracranial hemorrhage of 2 percent vs. 0.2 percent in the anticoagulation alone group.
To mitigate bleeding risk, the use of catheter-directed interventions is rising. Currently, catheter-directed interventions can be separated into two broader categories: catheter-directed thrombolysis (CDL) and catheter-based embolectomy (CBE).
CDL refers to the introduction of catheters directly into the affected pulmonary arteries with delivery of localized thrombolytic drugs. CDL is administered via ultrasound-assisted (USAT) and nonultrasound-assisted catheters.
USAT involves insertion of a dual-lumen catheter that simultaneously delivers thrombolytics and emits high-frequency, low-energy ultrasound. The ultrasound theoretically disrupts fibrin cross-linking to allow for improved thrombolytic penetration.
The most commonly used USAT device is the EKOSonic endovascular system (EKOS Corp, Bothell, WA).
In comparison to systemic thrombolytic trials, CDL investigations have been limited. Given the difficulty with powering these trials for clinical outcomes, the majority of trials have used surrogate markers, most commonly the ratio of RV to LV diameter (RV/LV ratio) measured on a long-axis view on either CT or transthoracic echocardiography.
In prior observational studies, RV/LV ratios of >0.9 have been independently associated with mortality at 30 days.13-15 Studied in the ULTIMA, SEATTLE and OPTALYSE trials, USAT effectively decreases the RV/LV ratio compared with anticoagulation alone.16-18
It is important to note that although many CDL trials have shown efficacy in reducing these short-term mortality surrogates, no CDL trials to date have directly shown short-term mortality benefit or prevention of hemodynamic decompensation.
Future research must compare CDL vs. systemic thrombolysis in a randomized fashion, USAT vs. non-USAT methods, and most importantly, whether the improvement of RV/LV ratios seen with CDL translate to clinically relevant outcomes.
CBE ranges in technique from simple maceration and aspiration to rheolytic thrombectomy to en bloc clot removal via small- and large-bore embolectomy devices.
In short, while maceration and rheolytic thrombectomy have been performed, these techniques are not recommended for routine use primarily due to adverse procedural complication profiles.
Regarding small-bore embolectomy, the Indigo Thrombectomy System (Penumbra, Inc, Alameda, CA) is an 8-French device that can be easily navigated into the pulmonary arteries.
This catheter houses a high-speed rotating rotor that creates negative suction for aspiration. While data on this device has yet to be published, results of the prospective single-arm trial involving this device with 119 patients showing a reduction in RV/LV ratios at 48 hours were recently presented (NCT 03218566).
Similarly, the FLARE trial examined the Flowtreiver device (Inari Medical, Irvine, CA).19 The Flowtriever is a large-bore device that engages a thrombus in the pulmonary artery with the deployment of three self-expanding nitinol disks which are then retracted back into the catheter while concurrent suction aspiration is applied.
This device is designed to avoid adjunctive thrombolytic therapy. The FLARE trial noted improvement in RV/LV ratios through a single-arm prospective trial of 106 patients.
Finally, the AngioVac device (AngioDynamics, Inc, Queensbury, NY) is a veno-veno bypass system designed with a filter that allows the operators to aspirate blood and return blood back into the body. This technology has commonly been applied to mobile thrombi in the caval systems or in the right atria in clinically compromised patients. Available systems are summarized in Table 1.
The benefits of catheter-directed interventions must be balanced by their potential harms. Systemic thrombolysis was complicated by a 6.3 percent risk of major bleeding and 2 percent rate of intracranial hemorrhage in the PEITHO trial, but demonstrated a roughly 3 percent absolute risk reduction in hemodynamic collapse.12
As stated earlier, CDL trials are limited to mostly single arm studies which, when combined, come with a decrease in all-cause mortality of 2.2 percent vs. 3.9 percent with anticoagulation alone in recent meta-analyses.8
However, CDL does carry higher rates of intracranial hemorrhage than anticoagulation alone (0.7 percent vs. 0.2 percent).2,8,20 Lower doses and lower durations may theoretically decrease the bleeding risks. However, the optimal balance of dose and duration have not been fully established and is an area of investigation.
As a general rule, it appears that large-bore CBE devices may carry elevated procedural risks of acute decompensation compared with CDL catheters.
The FLARE study reported three of 108 patients had intraprocedural hemodynamic or respiratory decompensation.19 When searching the Manufacturer and User Facility Device Experience (MAUDE) database of adverse events associated with medical devices received by the U.S. Food and Drug Administration (FDA), there were more instances of hemodynamic or respiratory decompensations associated with large-bore CBE compared with CDL.
While it appears these devices have the potential to cause more profound hemodynamic shifts, it is important to note these devices do not utilize thrombolytic therapy and thus are associated with lower risk of intracranial hemorrhage and other bleeding events than CDL.19

Only two devices have been cleared by the FDA: the EKOSonic USAT system and the Flowtreiver embolectomy device.
However, the FDA approval process has had a major impact on the landscape of PE device development and research. The FDA categorizes devices into three different classes: Class I devices (low risk to human health), Class II (moderate risk and can sufficiently provide assurance of safety and effectiveness of the device) and Class III (either present potential or unreasonable risk of illness or injury or insufficient information exists to provide reasonable assurance of safety and effectiveness).
Examples of Class I devices are elastic bandages, and wheelchairs, and pregnancy test kits are examples of Class II devices. Drug-coated balloons and transcatheter heart valves are examples of Class III devices, which undergo the most rigorous review process and often require large-scale randomized trial data proving efficacy and safety prior to approval.21
PE devices are categorized as Class II devices and have been evaluated through the 510(k) clearance pathway.22 PE devices primarily must demonstrate improvements in the RV/LV ratio with acceptable procedural safety profiles.
This use of the 510(k) clearance pathway as the primary mode of device evaluation has thus served as a major contributor to the lack of large-scale, randomized clinical trials to evaluate endovascular device performance in PE.
Risk Stratification and Treatment Strategy

In summary, escalation to invasive therapy is guided by the risk of the patient presenting with PE. In low-risk PE patients, the mortality rates are sufficiently low such that these patients should undergo anticoagulation alone.
They represent the majority of PE diagnoses. Intermediate-risk patients represent a heterogenous population and anticoagulation should still be initiated immediately. The data remain unclear with regard to when and which invasive strategies to employ in this group.
Whether these interventions translate to mortality improvement and improved quality of life in intermediate-risk patients has yet to be studied in a rigorous fashion.
Patients presenting with high-risk PE carry the highest mortality, but represent the minority of patients presenting with PE. Many require extracorporeal membranous oxygenation or isolated RV ventricular assist devices for stabilization with adjunctive systemic or catheter-directed therapies after acute support is instituted.
These recommendations are summarized in Figure 1. Given the complexity of patient-related and device-related considerations, PE response teams (PERT) have emerged across the country. These teams consist of multiple disciplines aimed to help navigate these challenging cases and consolidating knowledge and experience for individual hospitals.
PERT offers a team for consolidation of knowledge at the individual hospital level, patient advocacy on a patient level, and may offer the potential for improved research on a national level.
References
- Centers for Disease Control. Venous thromboembolism in adult hospitalizations - United States, 2007-2009. MMWR Morb Mortal Wkly Rep 2012;61:401-4.
- Giri J, Sista AK, Weinberg I. et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: A Scientific Statement From the American Heart Association. Circulation 2019;140:e774-e801.
- Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3145-6.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-9.
- Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016;48:780-6.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: Results of a multicenter registry. J Am Coll Cardiol 1997;30:1165-71.
- Becattini C, Agnelli G. Predictors of mortality from pulmonary embolism and their influence on clinical management. Thromb Haemost 2008;100:747-51.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414-21.
- Lin BW, Schreiber DH, Liu G, et al. Therapy and outcomes in massive pulmonary embolism from the Emergency Medicine Pulmonary Embolism in the Real World Registry. Am J Emerg Med 2012;30:1774-81.
- Secemsky E, Chang Y, Jain CC, et al. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med 2018;131:1506-1514.e0.
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010;170:1383-9.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010;55:2150-7.
- Schoepf UJ, Kucher N, Kipfmueller F, et al. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation 2004;110:3276-80.
- van der Meer RW, Pattynama PM, van Strijen MJ, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology 2005;235:798-803.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86.
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92.
- Tapson VF, Sterling K, Jones N, et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018;11:1401-10.
- Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: The FLARE Study. JACC Cardiovasc Interv 2019;12:859-69.
- Chatterjee S, Weinberg I, Yeh RW, et al. Risk factors for intracranial haemorrhage in patients with pulmonary embolism treated with thrombolytic therapy Development of the PE-CH Score. Thromb Haemost 2017;117:246-51.
- Learn if a Medical Device has been Cleared by FDA for Marketing. Available here. Last Accessed Dec. 11, 2018.
- Reclassification. Available here. Last Accessed Dec. 11, 2018.
Clinical Topics: Anticoagulation Management, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Cardiac Surgery and Heart Failure, Statins, Mechanical Circulatory Support, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, American Heart Association, Angiography, Coronary Angiography, Anticoagulants, Alloys, Blood Pressure, Brain, Echocardiography, Embolectomy, Fibrin, Fibrinolytic Agents, Heart-Assist Devices, Hemorrhage, Hypotension, Indigo Carmine, Intracranial Hemorrhages, Myocardial Infarction, Natriuretic Agents, Numbers Needed To Treat, Prognosis, Prospective Studies, Pulmonary Artery, Pulmonary Embolism, Quality of Life, Risk Assessment, Stroke, Thrombolytic Therapy, Suction, Tomography, Tomography, X-Ray Computed, Troponin, Ultrasonic Therapy, United States Food and Drug Administration, Aneurysm
< Back to Listings




