Peripheral Matters | Disparities in the Prevalence, Management and Outcomes of Peripheral Artery Disease
Peripheral artery disease (PAD) is a harbinger of atherosclerotic disease and considered to be a coronary artery disease equivalent.1 Individuals with PAD are at an increased risk of stroke, myocardial infarction and cardiovascular disease-related mortality.1-3 Moreover, complications from PAD itself, including critical limb ischemia (CLI) and lower extremity amputation (LEA), also cause substantial morbidity and mortality.4 Despite its serious consequences and prognostic importance, PAD continues to be underrecognized and undertreated.5 There are significant racial, socioeconomic and geographic disparities in the prevalence and outcomes of PAD, with Black Americans disproportionately affected.6
PAD and Black Americans
PAD in Black Americans
- PAD rate 2-fold higher than in non-Hispanic White Americans at all ages.
- CLI most likely presentation at PAD diagnosis compared with Whites.
- Subspecialist referral and care less likely. Less elective revascularization due to delayed presentation.
- Inferior revascularization outcomes, such as decreased limb salvage rates, increased graft failure and decreased secondary patency.
- Amputation rates 4-fold higher than in Whites.
The prevalence of PAD is twice as high in Black Americans compared with non-Hispanic White Americans across all age groups.6-8 This is particularly concerning because challenges inherent in studying the epidemiology of PAD have likely resulted in an underestimation of the true burden of disease. First, PAD is difficult to diagnose. Approximately 50% of patients with PAD are asymptomatic and only 10% experience typical claudication symptoms.7 Second, identifying cohorts of PAD patients that accurately capture the spectrum of diagnosis, management, outcomes and disease severity has historically been difficult.9 Relying on administrative data (such as insurance claims) for estimates of disease prevalence can lead to underestimation of the burden of disease because this source will not capture patients who have not presented with a clinical manifestation of PAD. Third, estimates of PAD prevalence may be further challenged in Black Americans, who are less likely to be referred to and see subspecialists for care.10-12 These limitations have also impacted the ability to generate high levels of evidence in guidelines for peripheral vascular intervention.13 Ongoing efforts to understand the drivers of the increased prevalence of PAD in Black Americans have shed light on several racial disparities.
Black individuals have some of the highest rates of tobacco use, hypertension and diabetes; however, treatment of these conditions is suboptimal at the population level.6 Tobacco use is strongly associated with the development and progression of PAD.6,7,14 Despite similar levels of interest in quitting compared with non-Hispanic White individuals, Black Americans have lower rates of smoking cessation.6 The discordance between the increased prevalence of PAD risk factors and inadequate medical management of these conditions exacerbates the PAD disparities observed in Black patients.
Furthermore, Black individuals are less likely to receive optimal medical therapy, such as statin therapy and antiplatelet agents, once diagnosed with PAD.6 Inadequate medical management represents a missed opportunity in PAD care and must be addressed as it likely influences progression of disease and increased rates of both CLI and LEA in Black Americans.
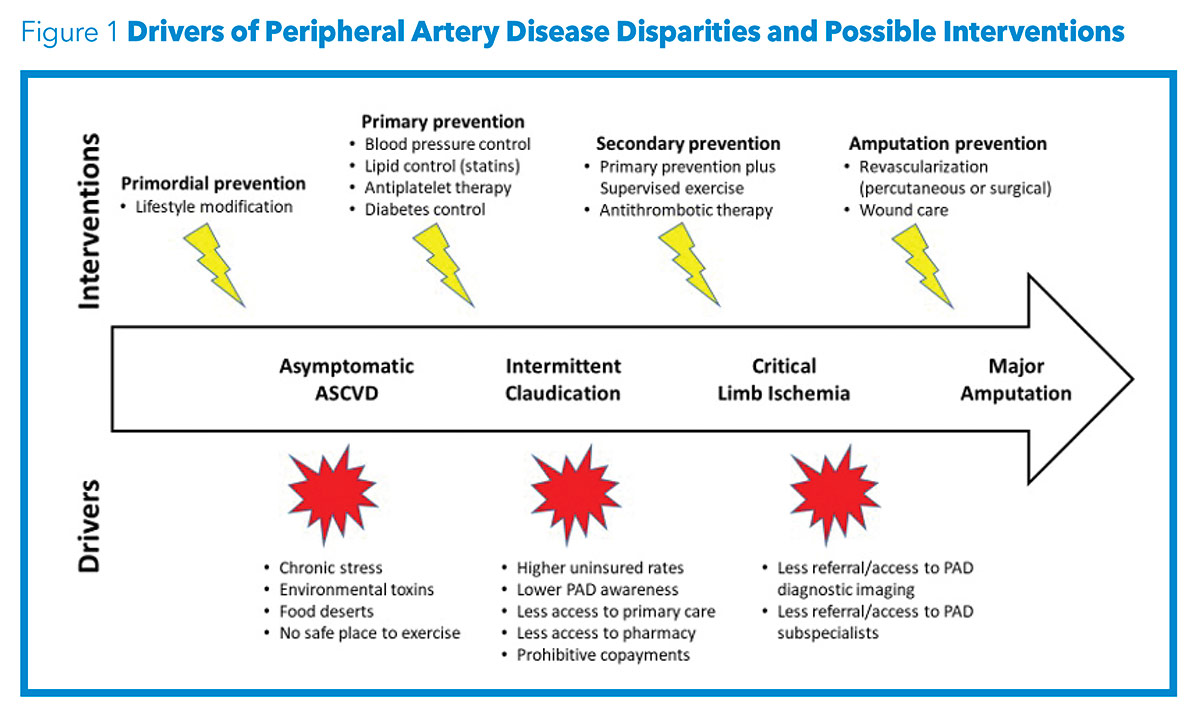
CLI, defined as ischemic lower extremity pain at rest, nonhealing wounds or gangrene, occurs with advanced PAD and is associated with a high risk of limb loss.7 Black patients are more likely to present with CLI at the time of PAD diagnosis compared with White patients.6,15,16 Potential explanations for the more severe and delayed presentations observed in Black patients with PAD include lack of access to primary and subspecialty care, differences in insurance status, inadequate medical management and lack of awareness of PAD. Delayed presentations result in less elective revascularization which may influence disease course.7,15 When revascularization does take place, Black race is associated with inferior outcomes, such as decreased limb salvage rates, increased graft failure and decreased secondary patency.15,17,18 Despite a national emphasis on reducing LEA rates, Black patients with PAD are more likely to undergo LEA than both open and endovascular revascularization procedures.6,19
Amputation is a catastrophic consequence of PAD associated with significant morbidity, mortality and health care costs.4,20 There are approximately 150,000 nontraumatic amputations per year in the U.S.21 Studies reported a decrease in the rate of amputation in the early 2000s but more recent data demonstrate a concerning trend towards increasing LEA rates once again.21
A recent statement from the American Heart Association highlights the importance of increasing public awareness of PAD and improving the use of evidence-based PAD therapies with the goal of reducing LEA rates by 20% by the year 2030.21 Unfortunately, amputation rates continue to be as much as four times higher in Black Americans compared with their White counterparts.6,7 Studies examining the relationship between race, socioeconomic status and geographic factors have shed light on specific communities with higher LEA rates among Black patients.
Certain communities within the U.S. are more vulnerable to PAD. In the U.S., rates of LEA are higher in the South Central and South Atlantic regions. Interestingly, these findings persist despite adjusting for clinical factors.22 Regions with higher rates of LEA tend to be clustered together with other regions of similarly high rates of LEA as opposed to regions with lower rates of LEA.22 The clustering phenomenon suggests local environmental factors may impact outcomes in specific communities. The contribution of such local factors to prevalence and outcomes in atherosclerotic vascular disease is highlighted by the geographic overlap between communities with high LEA incidence and the "Stroke Belt," a region in the Southeastern U.S. characterized by high stroke prevalence and poor outcomes.23
Possible factors influencing this regional disparity in rates of LEA and stroke include differences in access to both primary and subspecialty care, physician practice patterns, insurance status and increased prevalence of lower socioeconomic status within these regions. This is exemplified by higher rates of LEA among Black Americans in rural communities, which can be both geographically isolated and medically underserved.24
Notably, a significant proportion of patients with PAD live within urban environments. A recent study by Fanaroff, et al., found amputation rates were highest in metropolitan zip codes with a greater proportion of Black residents and lower socioeconomic status.25 Efforts to address PAD disparities in the U.S. may be more impactful if concentrated on these vulnerable regions.
The study of racial disparities in PAD is evolving as we stride for a more granular understanding of not only the differences between groups but the drivers of these disparities. Creative study designs are needed to successfully capture complicated factors such as socioeconomic status and environmental impact. Classically, the influence of socioeconomic status on medical outcomes has been evaluated with surrogate markers such as race, ethnicity and insurance status.26
The Distressed Communities Index (DCI) is a composite ZIP code-level score that combines unemployment, education level, poverty rate, median income, business establishments, job growth and housing vacancies.26 The DCI score ranges from no distress (score of 0) to severe distress (score of 100) and has been found to be an independent predictor of major adverse limb events.26 It will be important to continue to employ metrics such as the DCI to help us better understand the complex influences on disparities in PAD in vulnerable individuals and communities.
PAD and Hispanic/Latinx Populations
Patients of Hispanic/Latinx ethnicity with PAD may be similarly vulnerable to some of the disparities observed in Black Americans. Hispanics/Latinx are expected to comprise 31% of the U.S. population by the year 2060. Members of this ethnic group often have a high prevalence of cardiovascular and environmental risk factors.27 Despite having an increased burden of PAD risk factors, studies have noted a paradoxically lower prevalence of PAD in Hispanics/Latinx relative to other ethnicities.27
Coined the "Hispanic Paradox," this phenomenon has been observed in other cardiovascular diseases.28 It is possible that the prevalence of PAD among Hispanics/Latinx may be underestimated. Hispanics tend to have lower socioeconomic status and reduced access to health care relative to other ethnic groups, which increases the difficulty of diagnosing PAD.27
Similar to Black Americans, Hispanics/Latinx with PAD have been found to present with more advanced disease, have decreased revascularization procedures and have higher rates of LEA compared with White individuals.29,30 Exploring the drivers of the decreased prevalence of PAD among Hispanics/Latinx may yield useful information regarding protective and deleterious community, socioeconomic and environmental factors.
Moving to Solutions
Continuing to study racial disparities in PAD is imperative. CLI and LEA carry significant consequences not only for the individual patient but also local communities and broader society. While disparities research initially focused on the epidemiological evaluation of PAD, we are entering an era of action. Incorporating metrics such as the DCI score and evaluating issues in specific geographic regions has begun to provide both political and medical communities with specific targets for improvement.
We can also use disparities research to ensure that newer medications and procedures are making their way to underserved communities. While the disparities described herein are likely the result of both modifiable and nonmodifiable factors, health care providers must address what is modifiable. We eagerly await the evolution of disparities research toward more nuanced observational and interventional techniques leveraging implementation science and policy interventions to reduce disparities in health care outcomes for patients with PAD.



This article was authored by Carlos E. Barbery, MD, division of cardiovascular medicine, Perelman School of Medicine; Alexander C. Fanaroff, MD, MHS, division of cardiovascular medicine, Perelman School of Medicine, Penn Cardiovascular Outcomes, Quality, and Evaluative Research Center, Cardiovascular Institute; and Howard Julien, MD, MPH, ML, FACC, division of cardiovascular medicine, Perelman School of Medicine, Penn Cardiovascular Outcomes, Quality, and Evaluative Research Center, Cardiovascular Institute, and Penn Cardiovascular Center for Health Equity and Social Justice; all at the University of Pennsylvania.
References
- Subherwal S, Patel MR, Kober L, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol 2015;22:317-25.
- Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 1999;19:538-45.
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381-6.
- Cruz CP, Eidt JF, Capps C, et al. Major lower extremity amputations at a Veterans Affairs hospital. Am J Surg 2003;186:449-54.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317-24.
- Hackler EL 3rd, Hamburg NM, White Solaru KT. Racial and ethnic disparities in peripheral artery disease. Circ Res 2021;128:1913-26.
- Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol 2020;40:1808-17.
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509-26.
- Weissler EH, Zhang J, Lippmann S, et al. Use of natural language processing to improve identification of patients with peripheral artery disease. Circ Cardiovasc Interv 2020;13):e009447.
- Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation 2009;119:2463-70.
- Eberly LA, Richterman A, Beckett AG, et al. Identification of racial inequities in access to specialized inpatient heart failure care at an academic medical center. Circ Heart Fail 2019;12):e006214.
- Eberly LA, Garg L, Yang L, et al. Racial/ethnic and socioeconomic disparities in management of incident paroxysmal atrial fibrillation. JAMA Netw Open 2021;4:e210247.
- Sardar P, Giri J, Jaff MR, et al. Strength of evidence underlying the American Heart Association/American College of Cardiology Guidelines on Endovascular and Surgical Treatment of Peripheral Vascular Disease. Circ Cardiovasc Interv 2019;12:e007244.
- Clark D 3rd, Cain LR, Blaha MJ, et al. Cigarette smoking and subclinical peripheral arterial disease in Blacks of the Jackson Heart Study. J Am Heart Assoc 2019;8:e010674.
- Alshwaily W, Nejim B, Aridi HD, et al. Racial and gender disparity in aortoiliac disease open revascularization procedures. J Surg Res 2020;252:255-63.
- Soden PA, Zettervall SL, Deery SE, et al. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg 2018;67:549-556.e543.
- Jain AK, Kalbaugh CA, Farber MA, et al. Race and gender affect outcomes of lower extremity bypass. J Vasc Surg 2014;60:1275-81.
- Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation 2009;119:123-30.
- Newhall K, Spangler E, Dzebisashvili N, et al. Amputation rates for patients with diabetes and peripheral arterial disease: The effects of race and region. Ann Vasc Surg 2016;30:292-8.e291.
- Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005-2008. Prev Chronic Dis 2011;8:A141.
- Creager MA, Matsushita K, Arya S, et al. Reducing nontraumatic lower-extremity amputations by 20% by 2030: Time to get to our feet: A policy statement From the American Heart Association. Circulation 2021;143:e875-e891.
- Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000-2008. J Am Coll Cardiol 2012;60:2230-6.
- Howard G, Howard VJ. Twenty years of progress toward understanding the stroke belt. Stroke 2020;51:742-50.
- Minc SD, Goodney PP, Misra R, et al. The effect of rurality on the risk of primary amputation is amplified by race. J Vasc Surg 2020;72:1011-7.
- Fanaroff AC, Yang L, Nathan AS, et al. Geographic and socioeconomic disparities in major lower extremity amputation rates in metropolitan areas. J Am Heart Assoc 2021;10:e021456.
- Mehaffey JH, Hawkins RB, Charles EJ, et al. Socioeconomic "Distressed Communities Index" improves surgical risk-adjustment. Ann Surg 2020;271:470-4.
- Forbang NI, Hughes-Austin JM, Allison MA, Criqui MH. Peripheral artery disease and non-coronary atherosclerosis in Hispanics: another paradox? Prog Cardiovasc Dis 2014;57:237-43.
- Cortes-Bergoderi M, Goel K, Murad MH, et al. Cardiovascular mortality in Hispanics compared to non-Hispanic whites: a systematic review and meta-analysis of the Hispanic paradox. Eur J Intern Med 2013;24:791-9.
- Hua S, Isasi CR, Kizer JR, et al. Underuse of cardiovascular medications in individuals with known lower extremity peripheral artery disease: HCHS/SOL. J Am Heart Assoc 2020;9:e015451.
- Morrissey NJ, Giacovelli J, Egorova N, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. J Vasc Surg 2007;46:971-8.
Clinical Topics: Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Prevention, Atherosclerotic Disease (CAD/PAD), Nonstatins, Novel Agents, Statins, Interventions and Coronary Artery Disease, Hypertension
Keywords: ACC Publications, Cardiology Magazine, American Heart Association, Amputation, Benchmarking, Cardiology, Cardiovascular Diseases, Cluster Analysis, Coronary Artery Disease, Cost of Illness, Gangrene, Geography, Goals, Health Care Costs, Health Equity, Health Personnel, Housing, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hypertension, Implementation Science, Incidence, Insurance Coverage, Insurance Coverage, Limb Salvage, Lower Extremity, Medically Underserved Area, Myocardial Infarction, Pain, Platelet Aggregation Inhibitors, Policy, Poverty, Practice Patterns, Physicians', Prevalence, Prevalence, Prognosis, Referral and Consultation, Risk Factors, Rural Population, Schools, Severity of Illness Index, Smoking Cessation, Social Class, Social Justice, Socioeconomic Factors, Stroke, Tobacco Use, Unemployment
< Back to Listings


