Spotlight Series | Microvascular Dysfunction: Limitations of PCI in Patients With Small Vessel Disease
Significant atherosclerosis may be masquerading as ischemia with no obstructive coronary arteries (INOCA), which generally refers to symptoms or signs of ischemia in patients with <50% stenosis. Unfortunately, making the diagnosis of "nonobstructive" coronary artery disease (CAD) is more difficult than one would think.
Coronary angiography occasionally cannot optimally visualize a particular location, may have overlap of vessels or foreshortening of lesions, and provides information only on the contour of the coronary lumen (not the vascular wall that may contain substantial atherosclerosis with positive remodeling).
In addition, many studies have shown substantial inter- and intra-observer variability in determining the severity of stenosis, particularly in intermediate severity lesions and in large vessels. One study showed that both overestimation and underestimation of lesions with <60% stenosis were common.1
We know that atherosclerosis is a diffuse process and determination of stenosis severity is particularly difficult in long lesions or diffuse disease. Experienced cardiologists routinely underestimate the angiographic severity of diffuse disease, often due to the lack of a normal reference segment. Although traditional teaching used "% diameter stenosis" (comparing it to a "normal" reference segment) alone to determine lesion significance, fundamental physiology tells us that lesion length contributes greatly to the ischemic impact of any stenosis.2 Patients, particularly those with diabetes, may have diffuse disease or long lesions.
Fractional flow reserve (FFR) measurements with pullback recordings or intravascular imaging can be useful for optimal PCI within a given artery or to identify the need for CABG as an alternative to PCI. In the absence of advanced imaging or hemodynamics, it is likely that many patients are misdiagnosed as having "nonobstructive" disease.
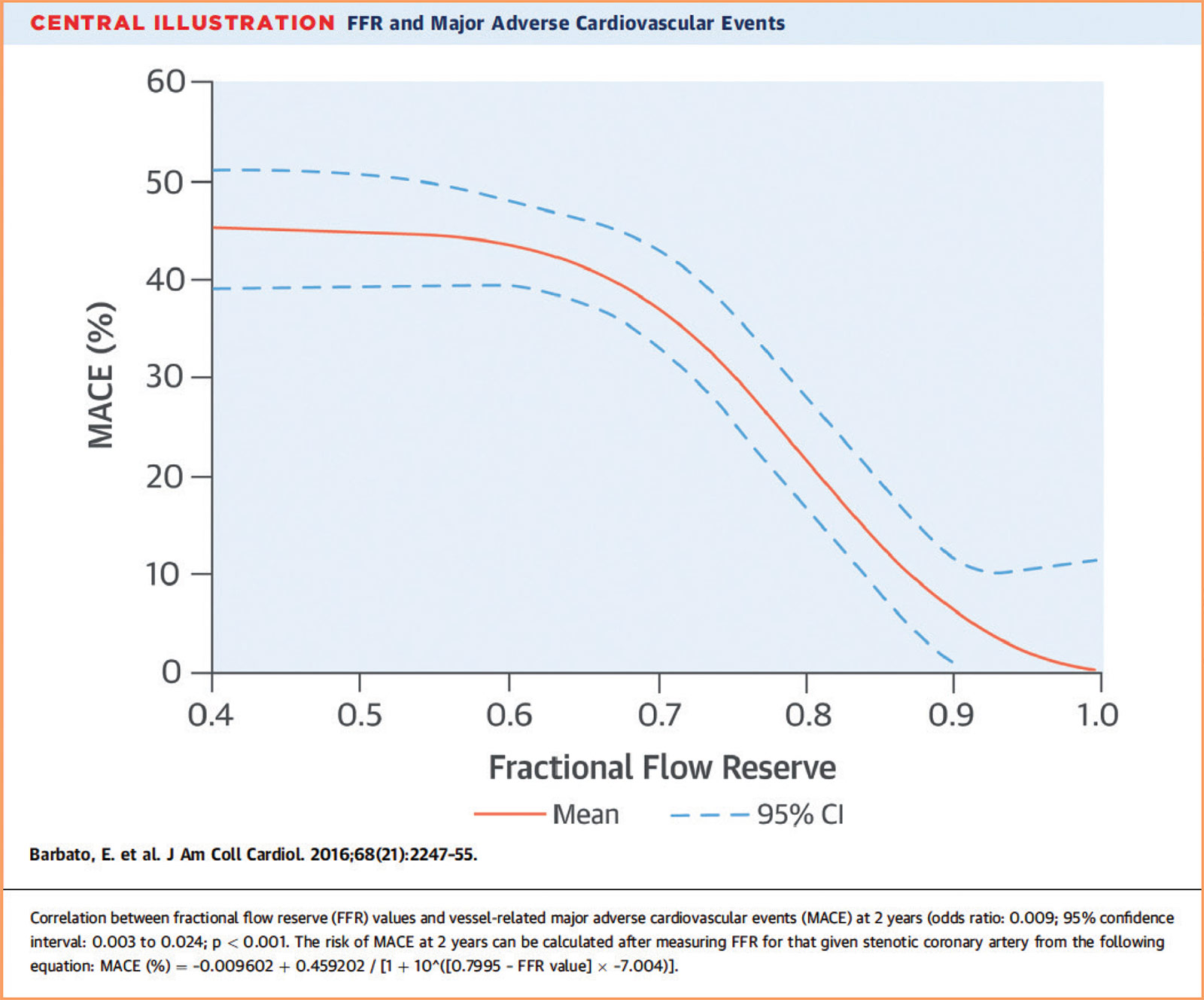
Although a cutoff of FFR <0.80 is used to determine whether a lesion benefits from PCI, the risk of future events based on FFR is a continuum. Both a meta-analysis and the subsequent FAME-2 trial found that patients with FFR values between 0.81-0.90 had significantly higher rates of major adverse cardiovascular events (MACE) than those with FFR values between 0.91-1.00.3,4 In fact, FFR values between 0.80-0.90 show event rates are "on the steep part of the curve" (Figure 1). Although it has not been proven that lesions with an FFR between 0.80-0.90 benefit from revascularization, these vessels are clearly higher risk, perhaps should be managed more aggressively, and should not be categorized as "nonobstructive."
Challenges in Determining Relative Contributions of Epicardial, Microvascular Disease
Microvascular disease may be difficult to diagnose and often coexists with epicardial coronary disease. Historically, intracoronary pressure measurements and FFR have been used to determine the hemodynamic significance of an epicardial coronary lesion, whereas coronary flow reserve measures the entire coronary tree. The index of microcirculatory resistance (IMR) is specific for microvascular disease. Since microvascular and epicardial coronary disease can both influence clinical outcomes,3-5 it is concerning that microvascular dysfunction could lower maximal hyperemic flow and therefore raise the FFR value, masking the severity of the epicardial lesion.
In cases of concern, it may be helpful to perform intravascular imaging to verify the severity of a stenosis. In a recent randomized trial of 1,682 patients with intermediate stenosis (40-70%), use of IVUS to guide revascularization was found to be noninferior to FFR with regard to death, myocardial infarction (MI) or revascularization at 24 months.6
IMR remains the gold standard as it is thought to be specific to the microvasculature and not affected by patient hemodynamics. IMR uses an intracoronary wire measurement during maximal hyperemia and seems to be accurate and reproducible in the absence of coronary stenosis. However, in the presence of significant epicardial stenoses, microcirculatory resistance may be overestimated unless incorporating coronary wedge pressure to account for collateral flow.7
PCI: Variable Impact on Microvascular Function
PCI may not correct microvascular issues, and occasionally it may transiently worsen microvascular function. It seems logical that if one improves proximal lesions, there will be better flow to the microvasculature. Despite the success of PCI in relieving a stenosis in the epicardial coronary artery, pre-existing microvascular dysfunction may inhibit sufficient myocardial blood flow.
Small Vessel Impact on Angina, Ischemia

The diagnosis of CAD has focused on presence of obstructive epicardial CAD. It is estimated that at least two of five male and female patients with angina referred for elective coronary angiography have nonobstructive epicardial coronary arteries, with rates relatively higher in women.
Missed the first article in the series, developed by invited Guest Editor, C. Noel Bairey Merz, MD, FACC? Click here to learn about the invasive and noninvasive diagnosis of small vessel disease.
This is particularly true in the setting of an acute MI, where transient microvascular dysfunction impairs maximal coronary hyperemia and may falsely elevate the FFR. Therefore, in this setting, a low FFR indicates true hemodynamic significance, but a normal FFR may not be definitive. Operators should consider using intravascular imaging and clinical judgement to determine the optimal treatment.
In a study of 50 patients undergoing elective left anterior descending artery stenting, elevated baseline pre-PCI IMR was not uncommon and a value ≥27 was independently associated with a 23-fold increased risk of developing periprocedural MI.6 This suggests that existing microcirculatory impairment may predispose toward microvascular plugging of embolized material after PCI.7
In a multicenter study, 572 patients with stable CAD underwent elective PCI followed by measurement of IMR immediately thereafter.8 Impaired microvascular function was defined as an IMR ≥25 (high IMR) and was present in 26% of patients post PCI. At long-term follow-up (median four years), the rate of MACE (a composite of death, MI and target vessel revascularization) was significantly higher in patients with high IMR (hazard ratio [HR], 1.56; 95% confidence interval [CI], 1.16-2.105; p=0.001). This was mainly driven by a difference in periprocedural MI (HR, 1.59; 95% CI, 1.11-2.28; p=0.004), which not surprisingly could increase post-PCI IMR. The authors suggest that IMR can be used to identify high-risk patients who might benefit from closer follow-up or therapies aimed at microvascular recovery. However, additional studies are needed to determine appropriate therapies post PCI.
It is likely that any adverse effects of PCI on vascular tone are transient. In a study of 142 patients with chest pain and no significant disease on angiography, 71 patients had a prior stent placed 17.1±17.1 months earlier. The authors reported coronary microcirculatory and epicardial vascular function were not significantly different from that of an age- and sex-matched population with similar symptoms.9
FFR May Not Normalize After PCI
It has been known for decades that post-PCI FFR is associated with worse outcomes. In the earliest registry study of 750 patients, 32% of patients had post-stent FFR <0.90; the six-month event rate was 20.3%.10 In 6% of the patients, FFR was <0.80 and the event rate was 29.5% (p<0.001) compared with FFR >0.90. The mechanism of residual abnormal FFR was unknown in this study.
DEFINE PCI, a multicenter, prospective study, performed blinded instantaneous wave-free ratio (iFR) measurement after angiographically successful PCI in 562 vessels in 500 patients.11 The mean baseline iFR value of 0.69±0.22 improved to 0.93±0.07 post PCI. Unrecognized residual ischemia after successful PCI was present in 24.0% of patients, with an iFR range of 0.60-0.89. Among patients with impaired post-PCI iFRs, 81.6% had untreated stenoses that were angiographically inapparent and 18.4% had diffuse disease. Most cases of residual ischemia were due to inapparent lesions potentially amenable to treatment with additional PCI, the authors concluded.
PCI For Tandem Lesions, Diffuse Disease and Small Vessels
Multiple lesions, overlapping stents and diffuse disease have been predictors of poor clinical outcomes. Often operators do not treat diffuse disease by PCI, despite theoretically improving inflow pressure if proximal lesions are treated. It is possible that by improving inflow, the microvasculature will also benefit. iFr may help determine which lesions could be considered.
In one study of tandem and diffuse coronary disease, iFR pullback predicted the physiological outcome of PCI with a high degree of accuracy.12 The predicted post-PCI iFR calculated online was 0.93±0.05; observed actual iFR was 0.92±0.06. Compared with angiography alone, the availability of iFR pullback altered revascularization procedural planning in nearly one-third of patients.
In the ORBITA study, blinded iFR pullback assessment was performed in 164 patients before randomization to PCI or medical therapy.13 Focal disease was defined as a unit drop ≥0.03 iFR within 15 mm, rather than over a longer distance. For focal vs. diffuse disease, no difference was seen in angina or quality of life post PCI. However, reduction of stress echocardiography ischemia was more effective post PCI for focal vs. diffuse CAD. These findings are not unexpected and suggest that patients with diffuse disease may have some symptomatic benefit from PCI.
PCI in small vessel disease (diameter ≤2.5 mm) is associated with increased risk of stent thrombosis and restenosis, likely due to reduced coronary flow and a smaller lumen to accommodate stent struts and neointimal hyperplasia that may occur during healing.
Guest Editor's Note: This informative article addresses epicardial coronary disease but "bypasses" the relevant small vessel problem. Specifically, coronary microvascular dysfunction is a "functional" issue of prearterioles (100-500 µm), and intramural arterioles (<100 µm) that cannot be treated with stenting. This is underscored by the recent NEJM publication not included here of the REVIVED trial among patients with severe ischemic left ventricular systolic dysfunction who received optimal medical therapy, in whom revascularization by PCI did not result in a lower incidence of death from any cause or hospitalization for heart failure.19
The ISAR-SMART randomized trial with 404 patients compared balloon angioplasty with bare metal stents (BMS) in small vessels.14 Angiographic restenosis rates were high in both groups (37.4% vs. 35.7%; p=NS). In the TAXUS V trial, angiographic restenosis (31.2% vs. 49.4%; p=0.01) and target vessel revascularization (10.4% vs. 21.5%; p=0.03) were lower but suboptimal in patients receiving first-generation paclitaxel drug-eluting stents (DES) compared with BMS.15 Fortunately, second-generation DES with improved antiproliferative agents, biocompatible polymers, thinner struts and smaller sizes have further improved outcomes in small vessels.
Drug-coated balloons (DCB) are emerging as an effective alternative treatment for small vessels. The BELLO trial assigned 182 patients to paclitaxel DCB or paclitaxel DES and reported a lower MACE rate in the DCB group at three years (15.4% vs. 38.9%; p=0.02) but no difference in the revascularization rate.16 The BASKET-SMALL 2 randomized study with 758 patients found that paclitaxel DCB was noninferior to paclitaxel or everolimus DES at one year (MACE, 7.5% for DCB vs. 7.3% for DES).17
Conversely, the Swedish Coronary and Angioplasty Registry (SCAAR) compared patients with small vessel disease treated with either paclitaxel DCB (n=1,154) or newer-generation DES (n=13,634). A propensity score-adjusted analysis showed a higher risk of clinical restenosis in the DCB group compared with the DES group at three years (4.1% vs. 1.8%; adjusted HR, 2.0; 95% CI, 1.537-2.674). There was no difference in lesion/stent thrombosis, MI and death.18
These results suggest clinical outcomes after PCI in small vessels are improving. Both new-generation thin-strut DES and DCBs may be responsible for the improvements in outcomes. Hopefully, the ability to manage epicardial disease regardless of lesion length or vessel size will ultimately result in symptomatic improvement in patients with combined macro- and microvascular disease.


This Spotlight Series article was authored by Cindy L. Grines MD, FACC, MSCAI, chief scientific officer and interventional cardiologist, and Pradyumna Tummala MD, FACC, FSCAI, cardiology section chief, both at Northside Hospital Cardiovascular Institute in Atlanta, GA.
References
- White CW, Wright CB, Doty DB, et. al. Does Visual Interpretation of the Coronary Arteriogram Predict the Physiologic Importance of a Coronary Stenosis? N Engl J Med 1984; 310:819-824
- Grines CL and Kern MJ. Should we use invasive fractional flow reserve (FFR), imaging or both to determine the significance of a "borderline" lesion? Catheterization and Cardiovascular Interventions 2022, 99(7):2016-2017
- Johnson N.P., Tóth G.G., Lai D., et. al.: Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014; 64: pp. 1641-1654.
- Barbato E, Toth GG, Johnson NP, et. al. A Prospective Natural History Study of Coronary Atherosclerosis Using Fractional Flow Reserve. JACC 2016;68:2247-2255
- AlBadri A, Bairey Merz CN, Johnson BD, et.al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women JACC 2019;73:684-693
- Koo BK, Kang JH, Jiang J, et. al. Fractional flow reserve or intravascular ultrasonography to guide PCI. N Engl J Med 2022, 387:770-789.
- Ng M, Yong A, Ho M, et. al. The Index of Microcirculatory Resistance Predicts Myocardial Infarction Related to Percutaneous Coronary Intervention. Circulation Cardiovascular Interventions. 2012;5:515–522
- Nishi T, Murai T, Ciccarelli G, et. al. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study., DOI: (10.1161/CIRCINTERVENTIONS.119.007889) 2019.
- Lim SH, Flammer AJ, Yoon MH, et. al. The Long-Term Effect of Coronary Stenting on Epicardial and Microvascular Endothelial Function. Circulation: Cardiovascular Interventions. 2012;5:523–529
- Pijls NH, Klauss V, Siebert U, et.al. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation. 2002;105(25):2950.
- Allen Jeremias A, Davies JE, Maehara A, et. al. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. J Am Coll Cardiol Intv. 2019 Oct, 12 (20) 1991–2001
- Nijjer SS, Sen S, Petraco R, Escaned J, et al. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386–1396.
- Christopher A. Rajkumar, Matthew Shun-Shin, Henry Seligman, Placebo-Controlled Efficacy of Percutaneous Coronary Intervention for Focal and Diffuse Patterns of Stable Coronary Artery Disease. Circulation: Cardiovascular Interventions. 2021;14:e009891
- Kastrati A, Schomig A, Dirschinger J, Mehilli J, Dotzer F, von Welser N, Neumann FJ. A randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. Circulation. 2000;102(21):2593-2598
- Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O'Shaughnessy CD, DeMaio S, Hall P, Popma JJ, Koglin J, Russell ME; TAXUS V Investigators. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. Jama. 2005;294(10):1215-1223.
- Latib A, Ruparelia N, Menozzi A, Castriota F, Micari A, Cremonesi A, De Felice F, Marchese A, Tespili M, Presbitero P, Sgueglia GA, Buffoli F, Tamburino C, Varbella F, Colombo A. 3-Year Follow-Up of the Balloon Elution and Late Loss Optimization Study (BELLO). JACC Cardiovasc Interv. 2015;8(8):1132-1134.
- Jeger R V., Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Schreiber M, Mahfoud F, Linke A, Stephan FP, Mueller C, Rickenbacher P, Coslovsky M, Gilgen N, Osswald S, Kaiser C, Scheller B; BASKET-SMALL 2 Investigators. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849-856.
- Silverio A, Buccheri S, Venetsanos D, Alfredsson J, Lagerqvist B, Persson J, Witt N, James S, Sarno G. Percutaneous Treatment and Outcomes of Small Coronary Vessels: A SCAAR Report. JACC Cardiovasc Interv. 2020;S1936-8798(19):32476-32478.
- Perera D, Clayton T, O'Kane PD, et al. N Eng J Med 2022;Aug 27:[Epub ahead of print].
Clinical Topics: Acute Coronary Syndromes, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Acute Heart Failure, Interventions and ACS, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, Acute Coronary Syndrome, Aneurysm, Metabolic Syndrome, Angiography, Heart Failure, Cardiology Magazine Spotlight Series
< Back to Listings