Cutting-Edge Structural Interventions | Transcatheter Tricuspid Valve Replacement: Useful or Not?

Tricuspid regurgitation (TR) is a common valvular disease estimated to affect >1.5 million people in the U.S., with a yearly incidence of about 200,000 and >300,000 patients in the U.S and Europe, respectively.1 Stated another way, a clinically significant form of this valvular disease affects 4% (or 1 in 25) of patients ages 75 and older.2
Despite the current guidelines favoring early tricuspid valve repair, especially when undergoing concomitant left-sided cardiac surgery, most patients with TR receive lifetime medical therapy until intractable right-sided heart failure (HF) and end-organ dysfunction appear.1 In recent years, multiple studies have drawn attention to the poor prognosis of untreated TR, and demonstrated a clear tendency of excess mortality in patients with increasing severity of TR, highlighting the need for interventional options to improve outcomes.3
TR associated with left-sided valvular or myocardial disease is often a marker for late-stage chronic HF with limited treatment options and an unfavorable overall prognosis. However, isolated TR is also independently associated with excess mortality if left untreated. Thus, TR represents an important public health problem with an unmet clinical need.
Surgical approaches, either repair or replacement, have been shown to carry a high risk of in-hospital mortality, reaching 10%, without significant improvement over the past 10 years.4 As transcatheter interventional options for valvular heart disease continue to expand, transcatheter tricuspid valve repair through leaflet approximation or annuloplasty has been investigated.
Despite studies showing promising results with greater survival and freedom from HF rehospitalization compared with conservative management, its use can be limited by anatomical infeasibility such as large leaflet coaptation gap, perforation, significant leaflet tethering with dense chordae, and leaflet impingement with a cardiac implantable electronic device lead.
A myriad of investigational transcatheter tricuspid valve replacement (TTVR) devices have emerged as an alternative to surgery and to address this outstanding need, with promising early results related to safety, efficacy and improvements in patients' functional status and quality of life.
Orthotopic Valve Replacement
Orthotopic TTVR is a form of valve replacement performed by implanting a bioprosthetic valve on an existing tricuspid annulus, in a fashion similar to how it is performed in transcatheter aortic or mitral valve replacement. Several investigational devices are available with a variety of access and anchoring systems.
EVOQUE
The EVOQUE system (Edwards Lifesciences, Irvine, CA) is a self-expanding bovine pericardial valve mounted on a nitinol frame with a fabric skirt delivered through a transfemoral approach. The perimeter is surrounded by nine anchors that allow stabilization through anchoring to the annulus, leaflets and chords of the tricuspid apparatus. Its compassionate use in 27 patients with severe symptomatic TR at inoperable or high surgical risk has demonstrated 92% success with no periprocedural mortality.5
The reduction in TR remained excellent at one year (≤moderate in 96% and ≤mild in 87%) with sustained symptomatic improvement (NYHA I/II in 69% compared with 11% at baseline).5 All-cause death and HF hospitalization at one year were both at 7% without any deaths being identified as device-related. An early feasibility trial of this device, TRISCEND, is ongoing with an early report of 56 patients demonstrating a similar success rate, reduction in TR and symptomatic improvement.6
Lux-Valve
The Lux-Valve (Jenscare Biotechnology Co., Ningbo, China) is a bovine pericardium valve with a self-expanding nitinol atrial disc, self-adaptive fabric skirt, two graspers and an interventricular septal anchor. It is deployed via a minimally invasive right thoracotomy and transatrial approach. First-in-human experience including 31 patients reported a 100% technical success rate with TR reduction to ≤moderate and ≤mild in 100% and 85.2%, respectively at one year. Symptomatic improvement has persisted at one year with 82.8% maintaining NYHA I/II and a 96.8% survival rate.7 A study investigating the second-generation LuX-Valve Plus delivered through transjugular access is currently underway (NCT05436028).
GATE
The GATE (NaviGate Cardiac Structures, Inc., Lake Forest, CA) device was the first TTVR performed and is composed of an equine pericardium tricuspid valve mounted on a nitinol alloy stent. Twelve right ventricular tines grasp the native leaflets and 12 right atrial winglets covered by microfiber polyester cloth provide a seal. It is delivered through a right thoracotomy and transatrial access. An early study including five patients reported 100% technical success, with all patients demonstrating TR reduction to ≤mild; however, one patient died before discharge.8
Cardiovalve
The Cardiovalve (Venus Medtech Inc., Hangzhou, China) is deployed through transfemoral access and consists of a bovine pericardial leaflet mounted on a dual nitinol atrial and ventricular frame creating 24 grasping points for anchoring. The atrial portion of the frame has a covered flange to allow sealing to prevent paravalvular leak. Its compassionate use has been reported in 15 patients demonstrating significant reduction in TR to ≤moderate in 100% and ≤mild in 8% at 30 days.9 An early feasibility study is currently underway (NCT04100720).
Intrepid
The Intrepid (Medtronic Plc, Minneapolis, MN) is a transfemoral system with a bovine pericardial valve housed within a nitinol dual stent frame. Early experience with three patients reported technical success in all, and an early feasibility study is currently recruiting patients with a goal to include 15 patients (NCT04433065).10
Heterotopic Valve Replacement
The orthotopic valve is not an all-comprehensive treatment. A certain proportion of patients with severe TR are deemed unfavorable for its implantation, mainly due to excessive annular dilatation exceeding the available device size, unfavorable device angle or approach, and severe right ventricular dysfunction. Heterotopic valves have been developed to overcome these issues by bicaval valve implantation allowing reduction of venous regurgitation and mitigating the symptoms related to severe right HF such as peripheral edema and renal and hepatic congestion.
Non-Dedicated Device
Initial experience has investigated the off-label use of the Sapien (Edwards Lifescience, Irvine, CA) balloon-expandable valve system implanted in the inferior vena cava after deploying an anchoring stent. Although the first-in-human study has demonstrated high technical success, a subsequent randomized controlled trial including 28 patients was terminated prematurely because four patients in the caval valve implantation arm required open heart surgery due to device dislodgement.11,12
TricValve
The TricValve (P+F Products + Features, Vienna, Austria) is a system designed specifically for caval implantation via a transfemoral approach and consists of two independent self-expanding bovine pericardial tissue valves, each separately designed to be deployed into the superior and inferior vena cavae, respectively. Individual patient experience has so far shown promising device success with symptomatic improvement (NYHA IV to II in one patient reported) up to one year.13 Early feasibility studies are currently being implemented in the U.S. (NCT03723239) and Europe (NCT04141137).
TRICENTO
The TRICENTO bicaval valved stent graft (New Valve Technology, Hechingen, Germany) is a custom-made system tailored to an individual patient's anatomy. It consists of a covered stent designed to land in the superior and inferior vena cavae through a transfemoral approach and contains a lateral bicuspid pericardial valve facing the right atrium allowing valvular function between the vena cavae and right atrium.
A multicenter retrospective study including 21 high-risk patients with severe TR has shown 100% technical success and no in-hospital mortality.14 Symptomatic improvement was observed, with 65% of patients demonstrating NYHA I/II at a median follow-up of 61 days compared with 5% at baseline.
Looking Ahead
Although studies investigating either orthotopic or heterotopic TTVR devices are limited in number to derive a definite conclusion, preliminary results appear to compare favorably with the surgical approach or transcatheter repair (Figure 1).15-17 In-hospital mortality is rarely seen with currently investigated TTVR devices as compared with the surgical approach, reaching up to 8.8-10%.4,18
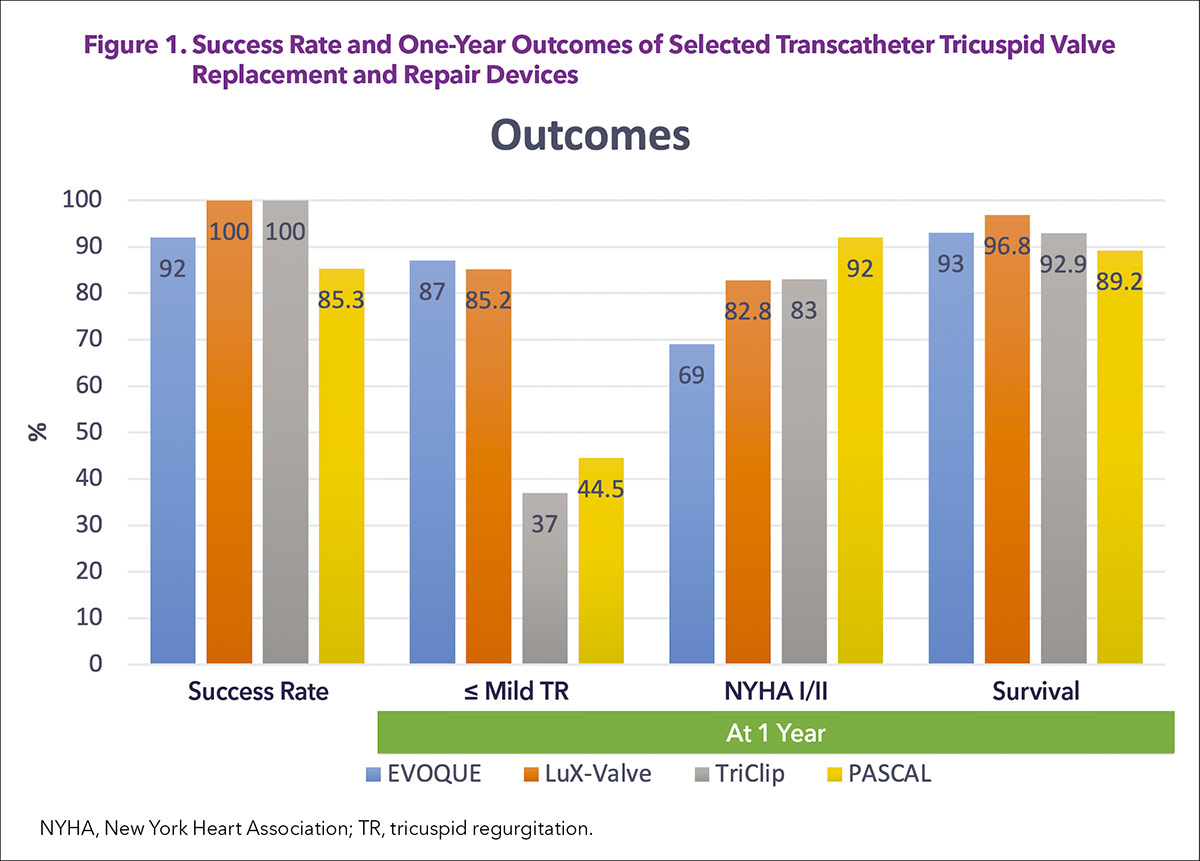
Mortality is comparable between transcatheter tricuspid repair and TTVR for up to one year, but a significant difference is seen in the reduction of TR, with TTVR reaching almost universal abolition of TR, which is sustained up to one year (≤mild TR: 85.2-87% in TTVR compared with 37-44.5% in transcatheter tricuspid repair).5, 7, 15,16 This difference can potentially expand further in the longer term since transcatheter edge-to-edge repairs are at higher risk of recurrence over time.
There are several challenges associated with the adoption of TTVR. TTVR may be a double-edged sword, allowing near complete abolition of TR, which in turn, however, can introduce afterload mismatch to the already dysfunctional right ventricle from long-standing TR, leading to early postperative hemodynamic compromise.
Further guidance on meticulous patient selection is imperative to avoid this complication. Moreover, the tricuspid valve and the right side of the heart have highly variable morphology and structure, including leaflet number, annulus size, and right ventricular and right atrial size, as well as its relationship with the inferior vena cava, each of which could affect the technical feasibility of valve deployment. Understanding the anatomical suitability of each valve system under investigation is crucial.
Lastly, the longevity of the valve implanted in the right side of the heart where pressures are significantly different from the left remains unknown. Optimal antithrombotic therapy to reduce valve thrombosis while minimizing bleeding complications should be investigated in the long term.
Despite these uncertainties, TTVR appears to be a promising approach, and a number of options are on their way for commercial use. The tricuspid valve is no longer a "forgotten" valve.


This article was authored by Hiroki Ueyama, MD, and Isida Byku, MD, FACC, from the Division of Cardiology, Emory Structural Heart and Valve Center, Emory University Hospital Midtown, in Atlanta, GA.
References
- Asmarats L, Puri R, Latib A, Navia JL, Rodés-Cabau J. Transcatheter tricuspid valve interventions: Landscape, challenges, and future directions. J Am Coll Cardiol 2018;71:2935-56.
- Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of Tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019;12:433-42.
- Taramasso M, Benfari G, van der Bijl P, et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol 2019;74:2998-3008.
- Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: Impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304-17.
- Webb JG, Chuang AM, Meier D, et al. Transcatheter Tricuspid valve replacement with the evoque system: 1-year outcomes of a multicenter, first-in-human experience. JACC Cardiovasc Interv 2022;15:481-91.
- Kodali S, Hahn RT, George I, et al. Transfemoral tricuspid valve replacement in patients with tricuspid regurgitation: TRISCEND study 30-Day results. JACC Cardiovasc Interv 2022;15:471-80.
- Modine T. Transcatheter tricuspid valve replacement with the LuX-Valve system 1-Year results of a multicenter FIH experience. Presented at TVT 2022, June 8, 2022.
- Hahn RT, George I, Kodali SK, et al. Early Single-site experience with transcatheter tricuspid valve replacement. JACC Cardiovasc Imaging 2019;12:416-29.
- Fam NP. Cardiovalve TTVR update. Presented at TVT 2022, June 8, 2022.
- Bapat VN. The INTREPID Valve for severe tricuspid regurgitation: First-in-man case experience. Presented at TCT 2020.
- Lauten A, Figulla HR, Unbehaun A, et al. Interventional treatment of severe tricuspid regurgitation: Early clinical experience in a multicenter, observational, first-in-man study. Circ Cardiovasc Interv 2018;11:e006061.
- Dreger H, Mattig I, Hewing B, et al. Treatment of severe tricuspid regurgitation in patients with advanced heart failure with CAval Vein Implantation of the Edwards Sapien XT VALve (TRICAVAL): a randomised controlled trial. EuroIntervention 2020;15:1506-13.
- Lauten A, Doenst T, Hamadanchi A, Franz M, Figulla HR. Percutaneous bicaval valve implantation for transcatheter treatment of tricuspid regurgitation: clinical observations and 12-month follow-up. Circ Cardiovasc Interv 2014;7:268-72.
- Wild MG, Lubos E, Cruz-Gonzalez I, et al. Early clinical experience with the TRICENTO bicaval valved stent for treatment of symptomatic severe tricuspid regurgitation: A multicenter registry. Circ Cardiovasc Interv 2022;15:e011302.
- Lurz P, von Bardeleben R, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 2021;77:229-39.
- Hahn RT. Transcatheter tricuspid valve repair:CLASP TR study one-year results. . Presented at EuroPCR 2022.
- Kodali S, Hahn RT, Eleid MF, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol 2021;77:345-56.
- Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953-60.
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Cardiac Surgery and VHD, Interventions and Structural Heart Disease, Interventions and Vascular Medicine
Keywords: ACC Publications, Cardiology Magazine, Heart Valve Diseases, Cardiac Surgical Procedures, Aneurysm, Transcatheter Aortic Valve Replacement, Tricuspid Valve
< Back to Listings

