Peripheral Matters | Dose-Reduced Direct Oral Anticoagulants: Practical Considerations

Direct oral anticoagulants (DOACs) are commonly used for the prevention of thrombosis in several cardiovascular contexts, including stroke prevention in patients with atrial fibrillation (AFib) and primary prevention, acute treatment or extended-duration secondary prevention of venous thromboembolism (VTE).1,2
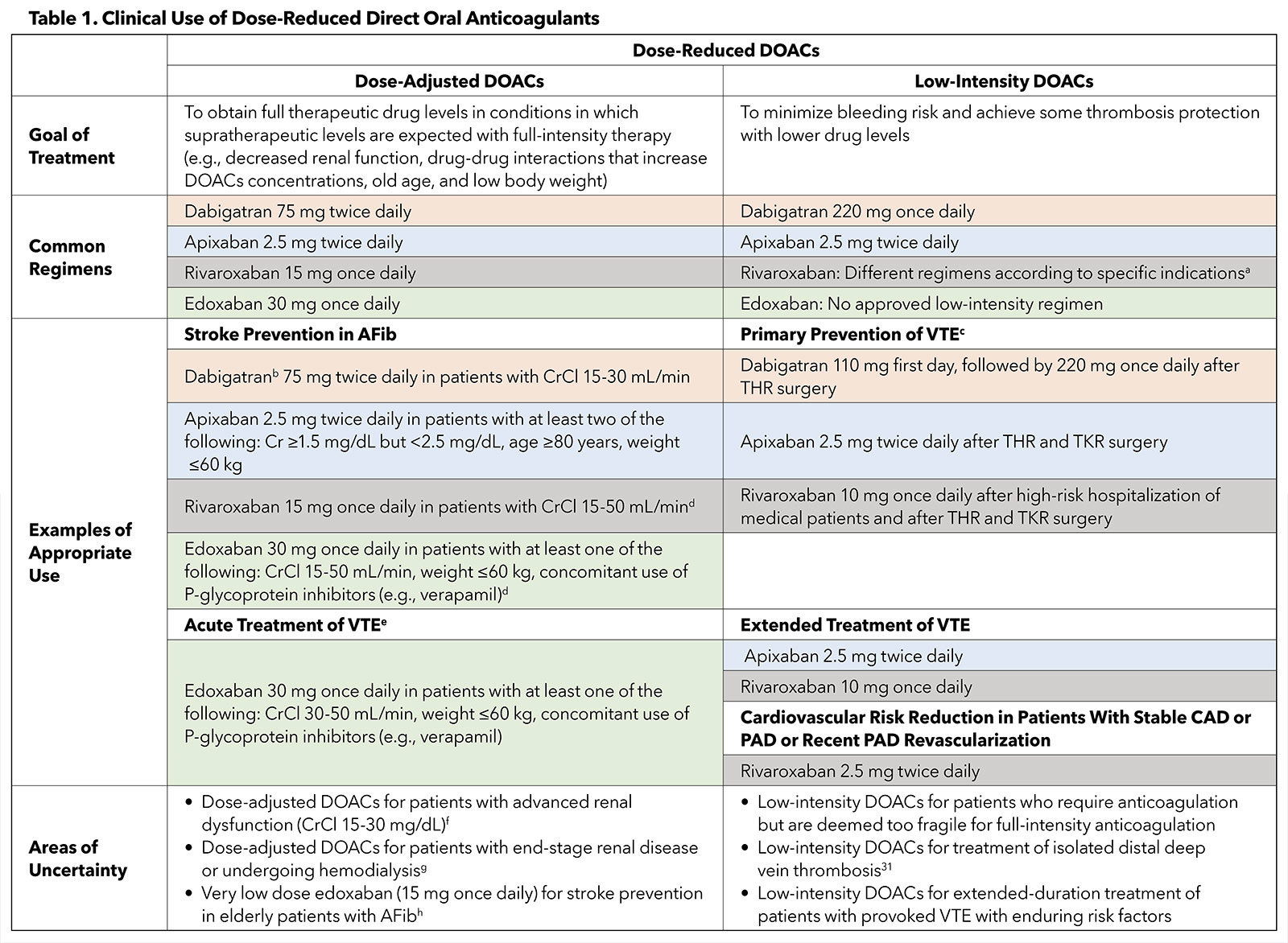
In several clinical scenarios, the DOAC dose is reduced.2 We briefly summarize hereafter the common regimens, appropriate use and uncertain circumstances related to dose-reduced DOACs (Table 1).
The reduction in the DOAC dose mainly has been investigated for two different purposes.2 One, dose adjustment to attain fully therapeutic plasma levels of the DOAC in conditions in which supratherapeutic levels are anticipated by full-dose therapy. Dose-adjusted DOACs have been primarily examined in randomized controlled trials (RCTs) to prevent stroke in specific subgroups of patients with AFib, such as patients with advanced age, low body weight, renal dysfunction, and/or drug interactions.3-6
Two, low-intensity DOAC therapy to diminish bleeding risk and provide some protection against thrombosis by using lower drug levels. This approach, in North America, has been largely evaluated for the prevention and extended-duration treatment of VTE, and for cardiovascular risk reduction in patients with stable atherosclerotic cardiovascular disease (ASCVD) or after recent revascularization for peripheral arterial disease (PAD).7-14
Indications For Dose-Adjusted DOACs

Stroke Prevention in Patients With AFib. The use of dose-adjusted DOACs in the subgroup of patients with AFib in the pivotal RCTs did not result in a significant interaction between dose adjustment and therapeutic effects of DOACs with respect to the primary clinical outcomes.3-6 Consequently, the U.S. Food and Drug Administration (FDA) approved dose-adjusted DOAC regimens for stroke prevention.
Subgroup analyses from pivotal AFib trials did not show an interaction between subgroups with vs. without dose adjustment and treatment effect of DOACs for primary clinical outcomes.3-6
The FDA-approved regimens are: 1) apixaban 2.5 mg twice daily in patients with AFib with at least two of these criteria: serum creatinine ≥1.5 mg/dL but <2.5 mg/dL, age ≥80 years, weight ≤60 kg; 2) rivaroxaban 15 mg once daily in patients with creatinine clearance (CrCl) 15-50 mL/min; 3) edoxaban 30 mg once daily in patients with AFib with at least one of these criteria: CrCl 15-50 mL/min, weight ≤60 kg, and concomitant use of P-glycoprotein inhibitors (e.g., verapamil).2
Dabigatran has been examined as a fixed dose in RCTs of patients with AFib, whereas the FDA recommended the dose of 75 mg twice daily to prevent stroke in patients with CrCl 15-30 mL/min.2 Except for some variation in patients with advanced kidney disease, these dosing recommendations by the FDA largely follow the dose adjustment criteria of the large pivotal trials.
Other Clinical Scenarios. Dose adjustment, i.e., reducing the dose with the intent of fully therapeutic levels, is not studied or approved for DOACs in other clinical scenarios, including acute treatment of VTE. In turn, RCTs for acute treatment of VTE excluded patients with serum creatinine >2.5 mg/dL or CrCl <25-30 mL/min.2 The only exception is edoxaban, for which dose adjustment from 60 mg once daily to 30 mg once daily has been tested in RCTs and approved by the FDA for short-term treatment of VTE in patients with any of these: CrCl 30-50 mL/min, weight ≤60 kg, and/or concomitant use of P-glycoprotein inhibitors.15,16
Indications For Low-Intensity DOACs

Primary Prevention of VTE. Low-intensity DOACs have been examined for primary prevention of VTE in high-risk outpatients with cancer, after high-risk hospitalization of medical patients, and after elective hip or knee arthroplasty. Rivaroxaban 10 mg once daily has been FDA-labeled for thromboprophylaxis after high-risk hospitalization of medical patients and after total hip or knee replacement surgery.7,11,17
Apixaban 2.5 mg twice daily can be used to prevent VTE in patients undergoing hip or knee arthroplasty,8,9 while dabigatran (110 mg first day, followed by 220 mg once daily) has been accepted for VTE prophylaxis after only hip surgery.10 Regarding high-risk ambulatory patients with cancers, low-intensity apixaban (2.5 mg twice daily)18 and rivaroxaban (10 mg once daily)19 have been considered for primary prevention of VTE by some guidelines20 but not thus far added to the FDA labels.2
Extended-Duration Treatment of VTE. Apixaban 2.5 mg twice daily12 and rivaroxaban 10 mg once daily,13 as low-intensity DOACs, have been demonstrated to be as effective as the full-intensity DOAC therapy for the extended-duration treatment of VTE, with numerically lower rates of the composite major and clinically relevant nonmajor bleeding events compared with full-intensity therapy. Thus, the FDA has approved these two low-intensity DOACs for this indication.2
Cardiovascular Risk Reduction in Patients With ASCVD. In patients with stable coronary artery disease, PAD,14 or recent revascularization for PAD,21 rivaroxaban 2.5 mg twice daily in combination with aspirin has resulted in favorable cardiovascular outcomes and has received FDA approval. On the other hand, in patients with recent acute coronary syndrome, rivaroxaban 2.5 mg twice daily, in combination with dual antiplatelet therapy, reduced the risk of cardiovascular and all-cause mortality but increased the risk of intracranial hemorrhage and major bleeding.22 Accordingly, rivaroxaban 2.5 mg bid has not resulted in FDA-labeled indications for patients with recent acute coronary syndrome, although it is approved in some European countries.2
Dose-Reduced DOACs in Routine Practice

Reports from large registries have indicated discrepancies between routine practice and evidence derived from RCTs with respect to the use of dose-reduced DOACs.2 For instance, non-recommended dose-reduced DOAC therapy was reported in 23.2% (2,423/10,426) of patients with AFib in the GARFIELD-AF registry.23 In the RIETE registry, among 4,716 patients receiving DOACs for acute VTE, 475 (10.1%) received a lower-than-recommended dose for acute treatment.2 Similar findings have been obtained from the Michigan Anticoagulation Quality Improvement Initiative registry, with off-label DOAC dosing described in 12.1% (438/3,617) of the participants.2
The disconnect between DOAC dosing in routine practice vs. randomized trials might be related to 1) lack of knowledge by treating clinicians, 2) obvious errors in routine practice encounters, or 3) decision-making for complex cases that have not been included in the existing RCTs. For instance, some clinicians administer low-intensity DOACs for fragile patients requiring anticoagulation who are at high risk of both thrombosis and bleeding.2 Moreover, the use of low-intensity DOACs has been reported in several observational studies for the treatment of isolated distal deep vein thrombosis, which has not been examined in RCTs.2
Other crucial areas of uncertainty in clinical practice are related to the intensity and duration of DOAC therapy in patients with provoked VTE with enduring risk factors,24 the efficacy and safety of DOACs for thromboprophylaxis in patients with mechanical valves, and the utility of DOACs for treatment of patients with left ventricular thrombus or heparin-induced thrombocytopenia.2
The use of dose-reduced DOACs in certain circumstances has been authorized with limited high-quality data. For example, although the FDA has allowed the use of dose-adjusted rivaroxaban and edoxaban for stroke prevention in patients with AFib and advanced kidney dysfunction (CrCl 15-30 mg/dL), the majority of RCTs have systematically excluded these patients.2
Nonstandard administration of dose-reduced DOACs beyond the well-defined indications for dose-adjusted or low-intensity therapy can lead to subtherapeutic drug levels and reduced efficacy.2 If possible, such nonstandard dosing should be performed under research protocols so that the practice generates knowledge for better future patient care.
Dose-Reduced DOACs in Special Populations

The effects of dose-reduced DOACs in specific patient groups deserve attention. Concerning patients with AFib undergoing hemodialysis, promising cardiovascular results have been reported in a small RCT (Valkyrie) for dose-adjusted rivaroxaban (10 mg once daily) compared with vitamin K antagonists.25 In contrast, apixaban 5 mg twice daily compared with warfarin was associated with numerically higher rates of cardiovascular mortality and nonmajor bleeding in patients with kidney failure and AFib.26
Regarding patients of Asian descent, although some small RCTs have used dose-reduced DOACs to prevent stroke in patients with AFib, the net benefits of dose-reduced vs. full-dose DOAC therapy in this population are yet to be clarified.2 In the ELDERCARE-AF trial, among Japanese patients with AFib aged ≥80 years who were deemed not suitable for full-dose anticoagulation, a very low dose of edoxaban (15 mg once daily) compared with placebo led to a lower risk of stroke and systemic embolic events without a significant increase in major bleeding.27 This interesting approach warrants further investigation, including in patients from other racial groups.
Although some unadjusted pooled estimates have indicated a possibility of sex differences in the efficacy and safety of DOACs,28 no major differences for dose-reduced DOACs have been noted in women vs. men to date. This issue deserves further investigation. It should be kept in mind that DOACs, including dose-reduced DOACs, are not approved for use during lactation and pregnancy due to limited safety data.2
Monitoring
DOACs do not require routine drug level monitoring.1,2 Laboratory monitoring of drug levels or alternative assays (diluted thrombin time for dabigatran and anti-factor Xa assays for the other DOACs) can be considered in particular cases, e.g., patients undergoing fibrinolytic therapy or urgent procedures.1,2
Periprocedural Interruption and Reversal
There is a paucity of evidence concerning the optimal perioperative management of dose-reduced DOACs.2 In steady conditions, the half-life of DOACs is not driven by the dose.2 Therefore, regardless of the dose intensity, it is reasonable to stop DOACs one day before and on the day of procedures with low bleeding risk. For procedures with higher bleeding risk, dose-reduced DOACs can be held two days before and on the day of the procedure without the need for anticoagulation bridging.2
Patients treated with dabigatran who have CrCl <50 mL/min require three to four full days of interruption prior to surgery, as the main clearance of dabigatran is through the kidneys.29 It is suggested to resume DOACs at least 24 hours after low-bleeding risk operations and 48-72 hours after high-bleeding risk operations.29
Reversal of DOACs using pharmacological agents (idarucizumab or activated prothrombin complex concentrate for dabigatran and andexanet alfa or 4-factor prothrombin complex concentrate for the other DOACs) is indicated in patients undergoing unexpected urgent surgery, or in those experiencing serious and life-threatening bleeding.2 Reversal is unlikely to be necessary for many other situations, e.g., when bleeding has stopped or can be managed with local measures, or when the procedure can be postponed for at least eight hours to allow the clearance of parts of the DOACs' effect.30 The optimal approach for the reversal of dose-reduced DOACs remains to be investigated.2
Conclusions
Dose-adjusted and low-intensity treatment are two different forms of dose-reduced DOACs with distinct objectives. Dose adjustment of DOACs has mainly been investigated for stroke prevention in patients with AFib and renal dysfunction, old age, low body weight, and/or drug interactions.
In contrast, low-intensity DOACs have been approved for primary or secondary prevention of VTE and cardiovascular risk reduction in patients with stable ASCVD (only rivaroxaban 2.5 mg bid). Dose adjustment according to AFib criteria should not be performed for patients with acute VTE.
Many scenarios of off-label use of low-intensity DOACs need further investigation in high-quality future studies.
- Low-intensity rivaroxaban 10 mg once daily for primary prevention and extended-duration treatment of VTE, and 2.5 mg twice daily for cardiovascular risk reduction in patients with stable ASCVD.
- Dabigatran has been examined as a fixed dose for stroke prevention in AFib, but the FDA has approved the adjusted dose of 75 mg twice daily in patients with CrCl 15-30 mL/min.2
- For primary prevention of VTE in high-risk ambulatory patients with cancers, low-intensity apixaban (2.5 mg twice daily) and rivaroxaban (10 mg once daily) have been approved by some guidelines,20 but not yet by the FDA.2
- The RCTs evaluated the adjusted dose of rivaroxaban and edoxaban in patients with AFib and CrCl 30-50 mL/min for stroke prevention; however, the FDA has also allowed these dose adjustments for patients with CrCl 15-30 mL/min.2
- The dose adjustment of dabigatran, apixaban, and rivaroxaban has not been approved for acute treatment of VTE.2
- Many RCTs for the use of DOACs excluded patients with advanced kidney dysfunction.2
- The exploratory Valkyrie RCT conducted in patients with AFib undergoing hemodialysis indicated a lower risk of composite fatal and nonfatal cardiovascular events with rivaroxaban 10 mg once daily compared to vitamin K antagonists.25
- Very low dose of edoxaban (15 mg once daily) compared with a placebo resulted in a reduced risk of stroke and systemic embolism without any significant increase in the major bleeding events in Japanese patients with AFib aged ≥80 years who were not regarded eligible for full-dose therapy (ELDERCARE-AF trial).27
AFib, atrial fibrillation; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; FDA, U.S. Food and Drug Administration; PAD, peripheral arterial disease; RCT, randomized controlled trial; THR, total hip replacement; TKR, total knee replacement; VTE, venous thromboembolism.



This article was authored by Sina Rashedi, MD, MPH, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran; Farbod Zahedi Tajrishi, MD, Department of Endocrinology, Emory University School of Medicine, Atlanta, GA; and Behnood Bikdeli, MD, MS, Cardiovascular Medicine Division and the Thrombosis Research Group, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, and the YNHH/Yale Center for Outcomes Research and Evaluation (CORE), New Haven, CT, and the Cardiovascular Research Foundation, New York, NY. Reach out to him on Twitter: @bbikdeli.
References
- Chen A, Stecker E, Waren BA. Direct oral anticoagulant use: A Practical guide to common clinical challenges. J Am Heart Assoc 2020;9:e017559.
- Bikdeli B, Zahedi Tajrishi F, Sadeghipour P, et al. Efficacy and safety considerations with dose-reduced direct oral anticoagulants: A review. JAMA Cardiol 2022;7;747-59.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104.
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or pci in atrial fibrillation. N Engl J Med 2019;380:1509-24.
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513-23.
- Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807-15.
- Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet 2007;370:949-56.
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75.
- Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708.
- Weitz JI, Lensing AWA, Prins MH, et al Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017;376:1211-22.
- Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319-30.
- Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615-24.
- Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86.
- Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711-9.
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;380:720-8.
- Streiff MB, Abutalib SA, Farge D, et al. Update on guidelines for the management of cancer-associated thrombosis. Oncologist 2021;26:e24-e40.
- Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020;382:1994-2004.
- Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9-19.
- Camm AJ, Cools F, Virdone S, et al. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol 2020;76:1425-36.
- Bikdeli B, Hogan H, Morrison RB, et al. Extended-duration low-intensity apixaban to prevent recurrence in patients with provoked venous thromboembolism and enduring risk factors: Rationale and design of the HI-PRO Trial. Thromb Haemost 2022;122:1061-70.
- De Vriese AS, Caluwé R, Van Der Meersch H, et al. Safety and Efficacy of vitamin k antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: A multicenter randomized controlled trial. J Am Soc Nephrol 2021;32:1474-83.
- Pokorney SD, Chertow GM, Al-Khalidi H, et al. Apixaban versus warfarin for stroke prevention in patients with end stage renal disease on hemodialysis and atrial fibrillation: Results of a randomized clinical trial assessing safety. Circulation 2019;140:E988-E9.
- Okumura K, Akao M, Yoshida T, et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020;383:1735-45.
- Zhou B, Wu H, Wang C, et al. Impact of Age, sex, and renal function on the efficacy and safety of direct oral anticoagulants vs. vitamin k antagonists for the treatment of acute venous thromboembolism: A meta-analysis of 22,040 patients. Front Cardiovasc Med 2021;8:700740.
- Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022;Aug. 22:[Epub].
- Levy JH, Ageno W, Chan NC, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:623-7.
- Bikdeli B, Caraballo C, Trujillo-Santos J, et al. Clinical presentation and short- and long-term outcomes in patients with isolated distal deep vein thrombosis vs proximal deep vein thrombosis in the RIETE Registry. JAMA Cardiol 2022;7:857-65.
Clinical Topics: Acute Coronary Syndromes, Anticoagulation Management, Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Anticoagulation Management and ACS, Anticoagulation Management and Venothromboembolism, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure
Keywords: ACC Publications, Cardiology Magazine, Anticoagulants, Arrhythmias, Cardiac, Acute Coronary Syndrome, Heart Failure, Secondary Prevention, Aneurysm, Venous Thromboembolism, Carotid Artery Diseases, Cardiovascular Diseases
< Back to Listings

