Cutting-Edge Structural Interventions | LAAO: An Effective Alternative With a Hidden Clinical Paradox

Oral anticoagulation (OAC) therapy has historically been the dominant strategy for stroke prevention for patients with atrial fibrillation (AFib). The 2023 ACC/American Heart Association (AHA) Guideline supports the use of OACs, preferring a direct oral anticoagulant (DOAC) over warfarin in patients with nonvalvular AFib.1 Despite the high efficacy of OAC therapy in stroke prevention, lifelong use is associated with challenges, including inappropriate dosing, nonadherence and bleeding risk.2 Finally, OACs are not suitable for every patient, such as those with contraindications to anticoagulation,1,3 or patients who suffer from stroke despite anticoagulation therapy (Figure 1).
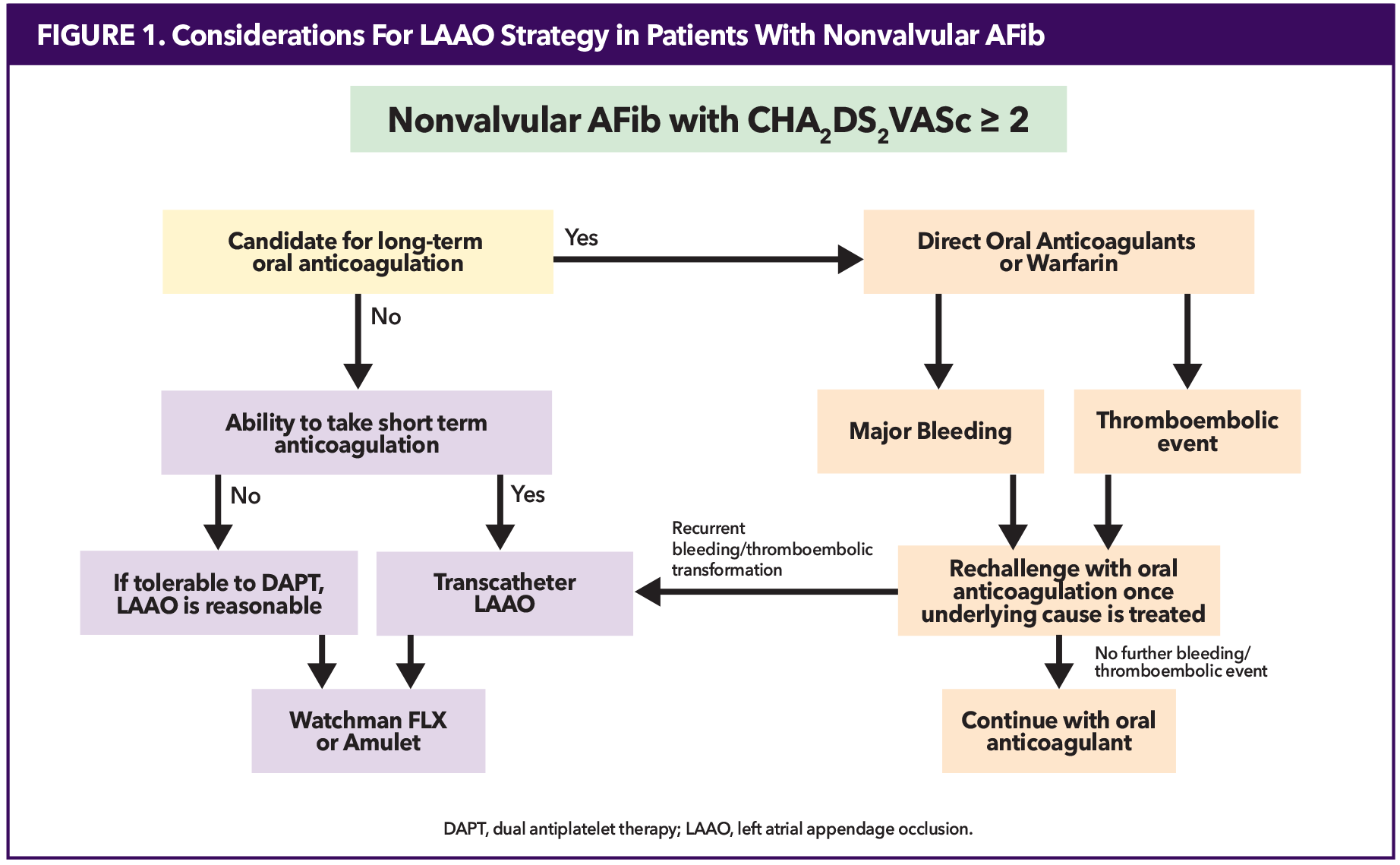
Since more than 90% of atrial clots form in the left atrial appendage (LAA),4 transcatheter LAA occlusion (LAAO) has become an attractive alternative in stroke prevention for patients with nonvalvular AF who have contraindications for OAC therapy.5
There are two Food and Drug Administration (FDA)-approved LAAO devices: the Watchman (Boston Scientific) and the Amulet (Abbott Vascular). The Watchman series (i.e., Watchman 2.5, Watchman FLX and Watchman FLX Pro) are plug-type devices with sizes ranging between 20-40 mm. The Amulet has a dual-closure mechanism with an inner lobe and sealing disc, with sizes ranging between 16-34 mm. The most recent randomized controlled trial (RCT) comparing the Amulet vs. the Watchman and Watchman FLX demonstrated comparable clinical outcomes, though the Amulet cohort showed lower peri-device leak (PDL) albeit at the expense of slightly higher procedural complications.6 As neither device has been proven superior to the other, device selection is dependent on patient anatomy and operator experience.
LAAO Guidelines and Patient Considerations
First approved for use in the U.S. in 2015, transcatheter LAAO is a stroke prevention technology primarily recommended for specific patient populations where long-term OAC poses challenges.7 According to the FDA, occlusion devices are approved for nonvalvular AFib patients who 1) have an indication for OAC for stroke prevention, 2) can tolerate short-term OAC, and 3) have an appropriate rationale to seek a nonpharmacologic alternative.
Current guidelines provide a moderate (Class IIa and IIb per ACC/AHA and Class IIb per European Society of Cardiology (ESC)) recommendations for LAAO for patients with contraindications to long-term OAC.1,3 Absolute contraindications to OAC, such as active bleeding or severe coagulopathy, are rare. However, most patients who undergo LAAO exhibit relative contraindications. These include prior major bleeding events, conditions that elevate bleeding risk, or significant frailty. Additional factors, including poor medication adherence, fall risk or lifestyle considerations, often provide the rationale for selecting LAAO. In line with FDA and clinical guidelines, shared decision-making is essential for determining the appropriateness of LAAO. Patients must be provided with evidence about their individual risks, the risks and benefits of OAC and LAAO, and the opportunity to discuss their preferences and values.
Registry Data
Within the first three years of approval in the U.S., over 38,000 LAAO procedures were performed across nearly 500 hospitals. Data from the NCDR highlight the dual burden of elevated stroke and bleeding risks in patient populations undergoing LAAO. The NCDR data also demonstrates excellent safety profiles for commercial use: in 98.3% of cases where device deployment was attempted, the device was successfully implanted. Major in-hospital adverse events (death, stroke, pericardial effusion, major bleeding and device embolization) hovered at 2%.5
Clinical Trials of LAAO vs. OAC
Currently, there are three RCTs comparing LAAO to OACs. PROTECT-AF and PREVAIL compared LAAO to warfarin, demonstrating noninferiority for stroke prevention and significant reductions in long-term bleeding risks.8,9 These findings support the safety and efficacy of LAAO devices in reducing stroke risk relative to OAC but not to DOAC.
PRAGUE-17 is the only RCT published that compares transcatheter LAAO with DOAC therapy.10 At the end of this trial and the four-year follow-up, transcatheter LAAO demonstrated noninferiority compared to DOAC therapy. Furthermore, LAAO was associated with a significantly reduced rate of nonprocedural clinically relevant bleeding.11 The clinical profile of the participants closely resemble the characteristics of patients in the NCDR, supporting the generalizability of the study's findings to current practice in the U.S.
After implantation, however, what therapies are needed after the procedure? Identifying these post-procedural challenges is discussed below (Figure 2).
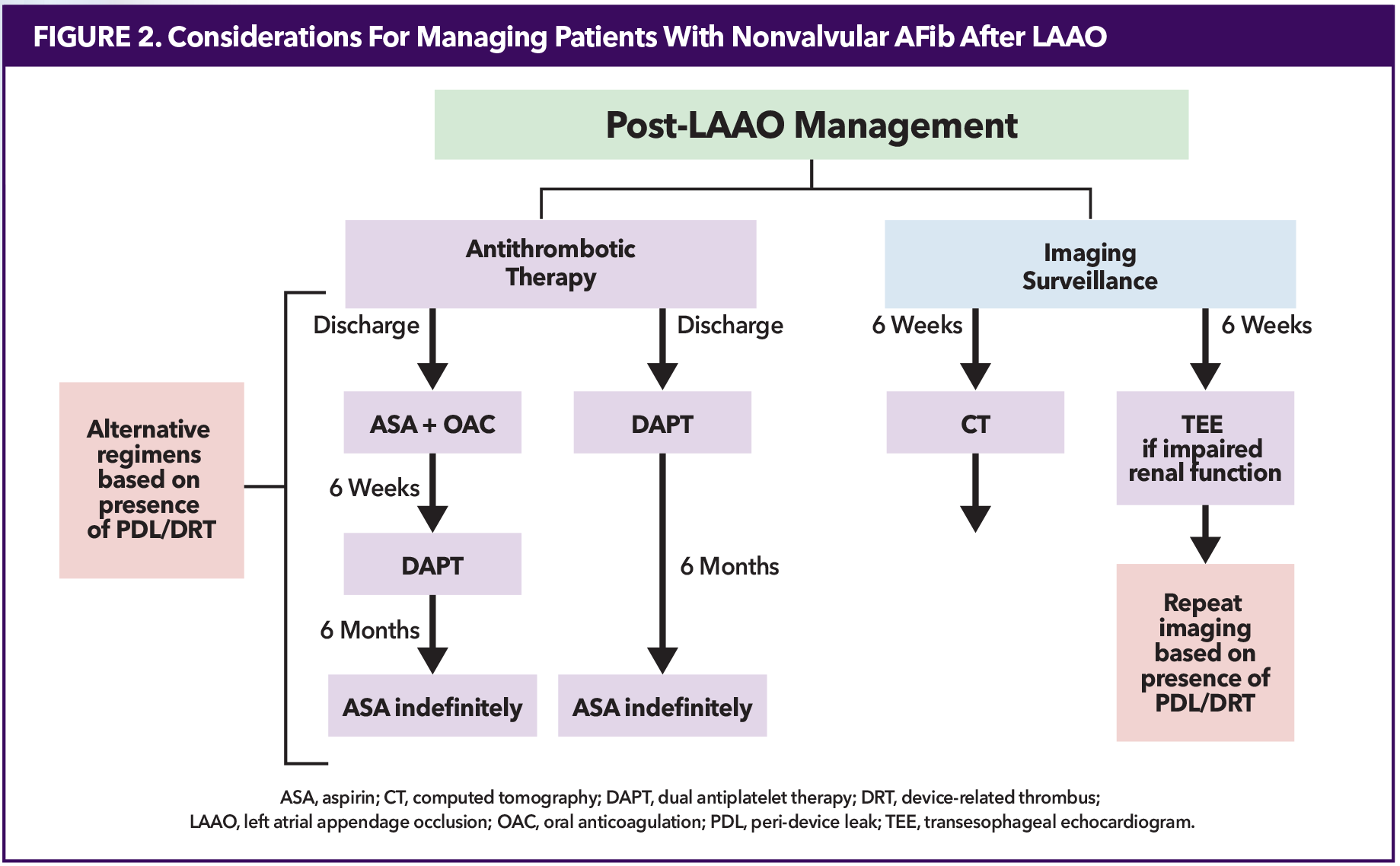
Post-LAAO Antithrombotic Therapy
Post-LAAO antithrombotic regimens, including OAC, dual antiplatelet therapy (DAPT) and long-term single antiplatelet therapy, vary greatly. ACC/AHA and ESC guidelines acknowledge this and do not provide recommendations.1,3
Patients with an Amulet device are discharged on DAPT (aspirin + clopidogrel) for six months. The Watchman FLX, initially approved for OAC + aspirin for six weeks, received FDA approval in 2022 for the alternative of DAPT post implantation, which is continued for six months. At six weeks, patients discharged with OAC + aspirin are transitioned to DAPT for 4.5 months. Based on patient characteristics and experience, clinicians may prefer OAC + aspirin or DAPT for Watchman devices. For both devices, lifelong aspirin is recommended after six months.
When a patient is transitioned from OAC + aspirin to DAPT or DAPT to aspirin monotherapy, imaging must be negative for PDL >5 mm and thrombus. If patients have a PDL >5 mm by imaging, whether detected periprocedurally or at follow-up, OAC + aspirin is most common. If the PDL resolves or becomes smaller, then patients are transitioned to DAPT. If not, then OAC + aspirin are continued.
There is even a report of long-term low-dose OAC (apixaban or rivaroxaban) use in Watchman patients.12 Remember that thromboembolic protection with OAC comes with the trade-off of higher bleeding risk compared to DAPT, making this an individualized decision between the clinician and patient.
This variability in post-LAAO regimens is simply because we do not have enough data to make a standardized decision. Trials addressing unique factors include CHAMPION-AF (NCT04394546), which evaluates DAPT, DOAC + aspirin or DOAC alone in patients with low-bleeding risk, and SAFE-LAAC (NCT03445949), comparing one vs. six months of DAPT and stopping vs. continuing therapy beyond six months.
Risks of LAAO
The two major complications of LAAO implantation are PDL and device-related thrombus (DRT).
Variability in LAA anatomy contributes to incomplete occlusion and leak post implantation. There is a lack of consensus on classification, detection methods and management of PDL. Leaks >5 mm need treatment, as mentioned above. Patients who cannot tolerate OAC continuation with >5 mm leak may opt for interventional PDL closure, including coils, plugs, occluders and radiofrequency ablation. Currently, PDL <5 mm is typically monitored without intervention. However, emerging data suggest even small leaks may increase thromboembolic risk.13,14 The PROTECT AF trial reported higher ischemic stroke risk in patients with 1-3 mm leaks compared to >3 mm leaks. Similarly, an NCDR registry analysis found leaks <5 mm were associated with increased thromboembolic events compared to no leak.
DRT increases thromboembolic risk four- to five-fold despite being relatively uncommon (approximately 4% incidence).15 Predictors of DRT include hypercoagulopathy, renal insufficiency (glomerular filtration rate 30-60 mL/min), permanent AFib, pericardial effusion and implantation depth >10 mm.16
While short-term anticoagulation is generally safe as an antithrombotic strategy, thrombus resolution often requires intensifying or extending the existing.4 Unlike PDL, which has alternative interventions, DRT management is largely limited to DOACs or warfarin with INR monitoring. Aspiration thrombectomy has been successfully performed in a small number of patients.17-19 The latest generation Watchman FLX Pro features a Hemocoat™ polymer coating demonstrating faster endothelialization in preclinical canine models.20 While its impact on human outcomes remains unproven, it may represent a step toward simplifying post-procedural antithrombotic management.

Such complications produce a clinical paradox, which challenges LAAO's role as an alternative to long-term OAC in patients with nonvalvular AFib. On one side, candidates for LAAO are patients who cannot tolerate long-term OAC. On the other is the fact that post-LAAO patients still require some form of either OAC or DAPT (albeit short term). Unfortunately, anticoagulation will be needed if a PDL or DRT occurs, possibly long-term.
Follow-Up Imaging
Post-LAAO surveillance is crucial for detecting PDL and DRT, yet optimal modality and timing remain debated. Transesophageal echocardiography (TEE) is the most common, typically performed at six weeks to three months and six months to one year. However, this schedule may not fully capture late-onset DRT or dynamic changes in PDL. DRTs have been detected beyond standard surveillance windows, sometimes incidentally during unrelated imaging studies,15 raising questions about whether extended or alternative surveillance strategies are warranted.
Cardiac computed tomography offers superior detection of PDL and DRT compared to TEE, providing better visualization of device endothelialization and healing.14 However, it is difficult to establish a universally accepted imaging protocol without definitive guidelines. As the adoption of LAAO increases, the growing patient population will provide a valuable opportunity to refine imaging protocols.
Conclusion
LAAO is a promising alternative to OACs for stroke prevention in patients who cannot tolerate long-term anticoagulation. As devices evolve, LAAO may be used in populations of patients with a wide range of LAA anatomies and no need for post-procedure OAC and DAPT. Currently, post-LAAO management and surveillance require further data and standardization.

References
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation. J Am Coll Cardiol 2024;83:109-279.
- Holmes DR Jr, Alkhouli M, Reddy V. Left atrial appendage occlusion for the unmet clinical needs of stroke prevention in nonvalvular atrial fibrillation. Mayo Clin Proc 2019;94:864-74.
- Van Gelder IC, Rienstra M, Bunting KV, et al. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2024;45:3314-414.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9.
- Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol 2020;75:1503-18.
- Galea R, De Marco F, Meneveau N, et al. Amulet or Watchman device for percutaneous left atrial appendage closure: primary results of the SWISS-APERO randomized clinical trial. Circulation 2022;145:724-38.
- Alkhouli M, Ellis CR, Daniels M, et al. Left atrial appendage occlusion: current advances and remaining challenges. JACC Adv 2022;1:100136.
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12.
- Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122-35.
- Osmancik P, Herman D, Neuzil P, et al. 4-Year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2022;79:1-14.
- Della Rocca DG, Magnocavallo M, Di Biase L, et al. Half-dose direct oral anticoagulation versus standard antithrombotic therapy after left atrial appendage occlusion. JACC Cardiovasc Interv 2021;14:2353-64.
- Alkhouli M, Du C, Killu A, et al. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the NCDR LAAO Registry. JACC Clin Electrophysiol 2022;8:766-78.
- Garg J, Kabra R, Gopinathannair R, et al. State of the art in left atrial appendage occlusion. JACC Clin Electrophysiol 2025;11:123-35.
- Alkhouli M, Busu T, Shah K, et al. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol 2018;4:1629-37.
- Simard T, Jung RG, Lehenbauer K, et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol 2021;78:297-313.
- Lai LKL, Alrayes H, Fram G, et al. Step-by-step ICE-guided aspiration thrombectomy: gastrointestinal bleeding patient with device-related thrombus on Watchman FLX. JACC Case Rep 2025;30:103219.
- Frisoli TM, Chiang M, Eng MH, et al. Percutaneous aspiration thrombectomy of thrombus attached to left atrial surface of a Watchman FLX device. JACC Clin Electrophysiol 2022;8(2):277-9.
- Vyas R, Kohler C, Pershad A. Percutaneous extraction of a large device-related thrombus on a Watchman™ device: a case report. Eur Heart J Case Rep 2022;6:ytab517.
- Saliba WI, Kawai K, Sato Y, et al. Enhanced thromboresistance and endothelialization of a novel fluoropolymer-coated left atrial appendage closure device. JACC Clin Electrophysiol 2023;9:1555-67.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Anticoagulation Management and Atrial Fibrillation, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Cardiology Magazine, ACC Publications, Atrial Fibrillation, Anticoagulants, Anticoagulation Management, Atrial Function, Left, Therapeutic Occlusion
