Focus on Heart Failure | HFpEF: Where We Stand in 2025
The incidence and prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing, along with the aging population and the ongoing rise in risk factors such as hypertension, diabetes and obesity. The diagnosis of HFpEF has been refined over time using more sensitive and specific diagnostic algorithms that have led to earlier diagnosis. In addition, our medical and device therapies for HFpEF have expanded over the years and now include several treatments with clinical benefits and more are in development.
Epidemiology of HFpEF
Nearly 6.7 million Americans live with HF with a per-person lifetime incidence estimated at 24%.1 Of these patients living with HF, more than half have HFpEF, or clinical HF with an LVEF estimated as >50%.2 This phenotype will affect nearly one in 10 patients by age 45 years with a population prevalence of 1-1.5%.2,3 HFpEF is associated with one-year mortality of 20-29% and frequent hospitalizations, including a 30-day all-cause readmission rate of 21%.1,2 As such, this HF subtype imposes tremendous economic burden on the health care system in addition to its impact on mortality, morbidity and quality of life of affected patients.
The reasons HFpEF is on the rise are multifactorial and include improvement in diagnosis as well as increases in the risk factors for cardiometabolic disease. Older age is a major risk factor for HFpEF, both through the direct impact of age-related myocardial stiffening and vascular dysfunction, and due to increasing medical comorbidity burden.4 The rising incidence of hypertension, diabetes and obesity directly mirror the rise in HFpEF.2,5,6
Obesity is associated with a unique phenotype of HFpEF associated with distinct structural and hemodynamic alternations compared to those with HFpEF and without obesity. This includes a greater degree of left ventricular remodeling, biventricular dilatation, and higher resting and exercise filling pressures, thought to be due expanded plasma volume through a variety of pathophysiologic mechanisms.7
It is also important to note race and sex differences regarding HFpEF epidemiology. The prevalence of HFpEF is higher in women than men.3 In the Framingham data cohort of patients with HF (n=6,340), a higher proportion of women had HFpEF compared to men in whom heart failure with reduced ejection fraction (HFrEF) was the predominant phenotype. This is postulated to relate to sex differences in ventricular remodeling in response to hypertension (i.e., concentric vs. eccentric) though further studies are needed.8,9
Race has been shown to play an important role interacting with sex. Black women experience the highest event rates for HFpEF hospitalization compared to other sex and race subgroups.3,10 In addition, underdiagnosis and higher rates of comorbidities such as hypertension, diabetes and obesity onset at younger ages may contribute to these racial differences in HFpEF.11
Diagnosis of HFpEF
A recently proposed "Universal Definition of Heart Failure" describes HF as a clinical syndrome characterized by signs and/or symptoms of HF due to structural and/or functional cardiac abnormalities, further supported by the presence of objective evidence of cardiogenic congestion including elevated biomarkers (e.g., natriuretic peptides) and/or as demonstrated through diagnostic testing.12 Using the above definition, HF is further classified by EF, with HFpEF distinguished by an EF ≥50%.
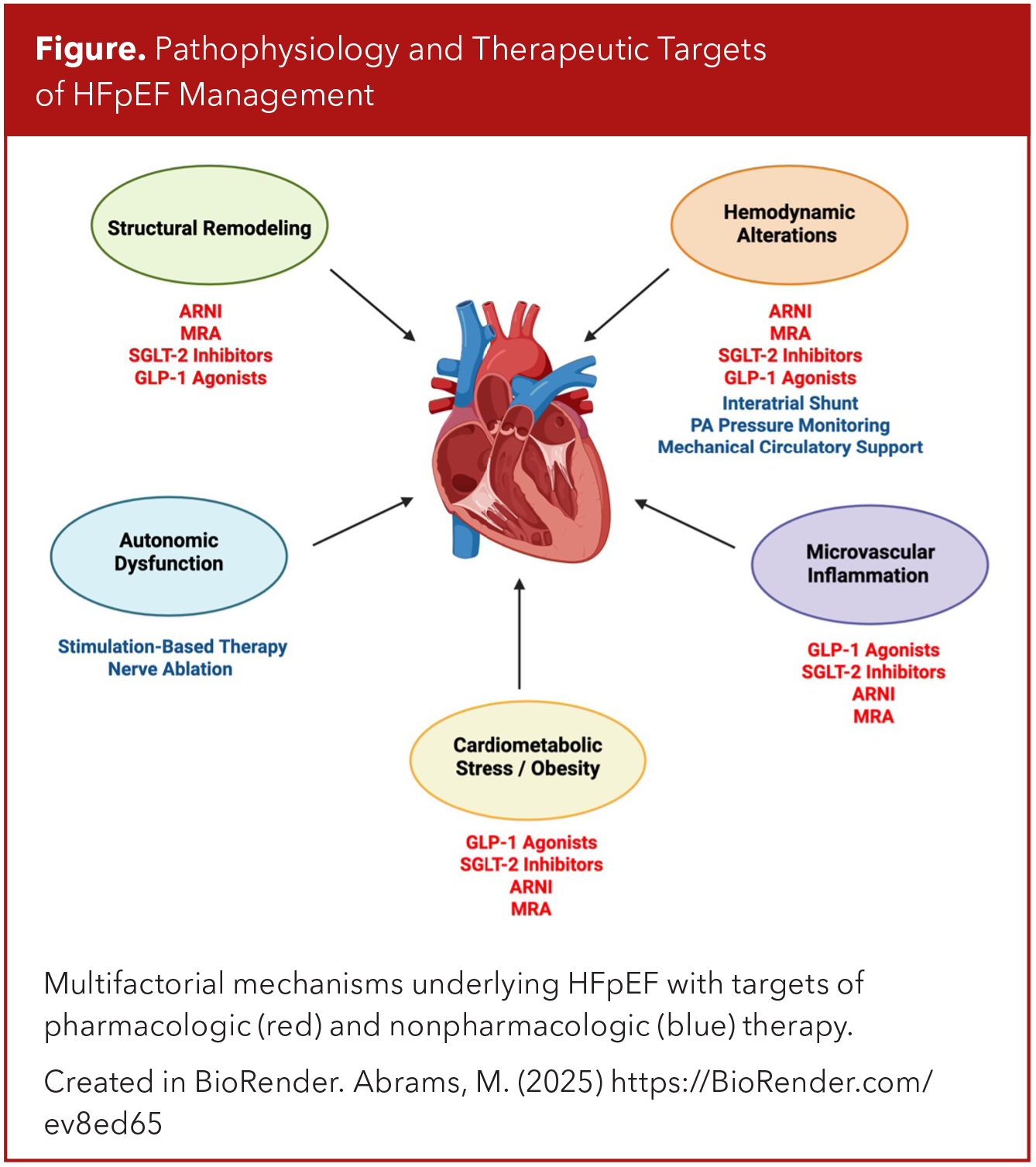
Though defining HF using the above criteria may appear straightforward, the process of diagnosis HFpEF is often less clear. The unique and multifactorial pathophysiologic mechanisms of HFpEF result in a wide spectrum of phenotypes/presentations, and in some cases initially nondiagnostic findings, such as normal natriuretic peptide levels (Figure).3,13,14 As such, several diagnostic algorithms have been developed to aid the assessment of cases where the probability of a HFpEF diagnosis is less certain.
The main two diagnostic scoring systems are: H2FPEF and HFA-PEEF.15,16 The H2FPEF algorithm utilizes patient demographics/clinical characteristics (age, BMI >30 kg/m2, history of hypertension or atrial fibrillation) as well as echocardiographic data (E/e' ratio >9, PASP >35 mmHg) to generate a score from which the likelihood of having HFpEF can reasonably be predicted.15
The HFA-PEEF is a more involved scoring system, utilizing many echocardiographic features, both functional (E/e' ratio ≥9, tricuspid regurgitation velocity >2.8 m/s, global longitudinal systolic strain <16%) and morphologic (left atrial volume index >29, left ventricular mass index >115/95 g/m2, relative wall thickness > 0.42, left ventricular wall thickness >12 mm), as well as cardiac biomarker (natriuretic peptide) levels, divided into "major" and "minor" criteria, to generate a score.16 In both algorithms, score thresholds are statistically determined, above (or below) which a diagnosis of HFpEF can be made with reasonably high (or low) confidence. These tests, however, are not without limitations, and in cases of diagnostic uncertainty due to indeterminate scores, further testing may be indicated.
Both noninvasive and invasive testing may be used to provide more a definitive diagnosis, and testing is often performed with exercise, as one unique feature of HFpEF is that patients may exhibit normal filling pressures at rest upon initial diagnostic assessment, which only then become elevated with stress.17,18 Noninvasive testing can be performed using stress echocardiography, using similar parameters (E/e' ratio ≥15, TR velocity >3.4 m/s) as aforementioned. Invasive hemodynamic evaluation with right heart catheterization both at rest (LVEDP >16 mmHg, PCWP >15 mm Hg) and with exercise (PCWP >25 mm Hg) remains the "gold standard," though it is typically reserved as a later step in instances of diagnostic uncertainty given its invasive nature and potential resource constraints and accessibility limitations.3,13,14
Once a diagnosis of HFpEF is reached, guideline-directed medical therapy should be initiated to target the various mechanisms underlying HFpEF.
Medical Therapy For HFpEF: Four Pillars?
Until approximately 2019, evidence-based medical therapy for HFpEF was limited compared to that of HFrEF, with diuresis as the only major treatment option. However, over the last several years, effective medical therapies for HFpEF have been established demonstrating significant improvements in patient-centered outcomes in those affected by HFpEF.
SGLT2 inhibitors for example, have demonstrated clinical benefit in two large, randomized trials in patients with HFpEF (EF ≥40%). In EMPEROR-PRESERVED studying empagliflozin and in DELIVER studying dapagliflozin, there was a significant reduction in cardiovascular death and HF hospitalizations compared to placebo, driven mostly by reductions in HF hospitalizations.19,20
The mechanisms of SGLT2 inhibitors are multifactorial and related to a diuretic effect, improved myocardial and vascular metabolism leading to lower preload, reduced afterload, and improvements in cardiac structure and function.21 At present, SGLT2 inhibitors have a class 2a recommendation in patients with HFpEF.22
Mineralocorticoid receptor antagonists (MRA) have also demonstrated clinical benefit in patients with HFpEF. TOPCAT studied the impact of spironolactone on cardiovascular death, cardiac arrest and HF hospitalizations in patients with HFpEF. Although the overall study findings were neutral, when patients enrolled in Russia and Georgia who likely did not have HFpEF were excluded, there was a significant reduction in the primary outcome among patients in the Americas.23,24
New "HF in a Box" Equips Clinicians With Comprehensive HF Tools
ACC's comprehensive resource, "HF in a Box," supports clinicians manage heart failure (HF). Educational materials in the global toolkit – including facilitator and participant guides, infographics and videos – focus on helping clinicians integrate SGLT2 inhibitors into HF therapy as part of guideline-directed medical therapy. This free resource is free and available in both English and Spanish. Learn more here.
More recently, FINEARTS-HF demonstrated reductions in cardiovascular death and HF events with finerenone, a nonsteroidal MRA, compared to placebo in >7,400 patients with HFpEF.25 Notably, this effect was seen despite the use of SGLT2 inhibitors. No head-to-head study has compared spironolactone and finerenone to date. Currently, MRAs have a 2b recommendation for the treatment of HFpEF, though this may be changed with the FINEARTS-HF trial results.
Angiotensin receptor-neprilysin inhibitors (ARNIs) are another component of medical therapy for HFpEF. PARAGON examined the impact of sacubitril-valsartan in with HFpEF (EF ≥45%) compared to valsartan.26 While the trial did not demonstrate a clinically significant benefit in reduction in HF hospitalization or cardiovascular death (hazard ratio, 0.87, 95% CI, 0.75-1.01), certain clinical subgroups including women and those with an EF on the lower side of the spectrum of EF demonstrated greater clinical benefit. It is postulated that women have more arterial stiffening with aging that sacubitril-valsartan may more effectively counter or that natriuretic peptides, which are generally lower in women, are more impacted by the medical therapy and may therefore lead to a stronger effect.27 Due to these data, ARNI currently has a 2b recommendation in patients with HFpEF.
The newest exciting development in the way of medical therapy for HFpEF has been medications that target obesity such as incretin mimetic-based therapies including GLP1 receptor agonists. Incretin mimetics, which include the GLP1 receptor agonist semaglutide and the GLP1/glucose-dependent insulinotropic polypeptide (GIP) receptor co-agonist tirzepatide, are anti-obesity medications associated with substantial and sustained weight loss in patients with obesity by delaying gastric emptying thereby reducing appetite and food intake.28,29
While not associated with clinical benefit in HFrEF, recently, these drugs have been studied in patients with HFpEF demonstrating improvements in clinical outcomes such as health-related quality of life (STEP-HFpEF) as well as reductions in cardiovascular death and HF events (SUMMIT).30,31 Secondary analyses from these large randomized trials suggest that weight-loss induced reduction in plasma volume as a key mechanism for clinical benefit, though further studies are needed.32 Additionally, the impact on skeletal muscle mass and quality will be important to establish given the potential for sarcopenia in this HF population.33 While these trials are more recent than the last iteration of HF guidelines, it is anticipated that these anti-obesity therapies will be recommended to treat patients with obesity and HFpEF.
Device-Based Therapies For HFpEF
Although in recent years several studies have demonstrated the benefit of pharmacologic treatments for HFpEF, there remains an unmet clinical need in HFpEF therapy. Device-based approaches that target various pathophysiologic processes in HFpEF have emerged as potential options to fill in these therapeutic gaps.34
Ambulatory pulmonary artery (PA) pressure monitoring systems have been developed as a means of objectively assessing volume status in the outpatient setting to closely titrate HF medications, and in particular, diuretics. The CardioMEMS HF System features the use of an implanted sensor in the PA that directly measures filling pressures, and its utility in management of HF has been studied in three major randomized control trials.35-37 The pivotal CHAMPION trial indicated that use of PA pressure monitoring in patients with HF results in reduced rates of HF hospitalizations, and the subgroup analysis of HFpEF resulted in similar outcomes.35,36
While the subsequent GUIDE-HF trial did not find a difference in cardiovascular outcomes using PA pressure monitoring, a newly published trial based out of Europe, MONITOR-HF, demonstrated findings similar to the earlier CHAMPION trial – that PA pressure monitoring to guide HF management reduced HF-related hospitalizations and improved quality of life.38 The CardioMEMS HF System is now approved by the U.S. Food and Drug Administration in the treatment of HF, and PA pressure monitoring is now considered a class 2b recommendation per the 2022 ACC/AHA guidelines.39
An additional therapy that has been studied for use in HFpEF is the interatrial shunting device (IASD), which aims to relieve left atrial pressure through the artificial creation of connection between atria (or in some approaches between the left atrium and coronary sinus).40,41 Although the first major randomized clinical trial for this approach – REDUCE LAP-HF II – demonstrated no difference in cardiovascular outcomes between the group treated with IASD vs. a sham procedure, a post hoc analysis revealed a large subgroup of "responders" in whom potential efficacy was demonstrated. This included those with peak exercise pulmonary vascular resistance <1.74 Woods units.41,42 The RESPONDER-HF studying this particular subgroup is ongoing (NCT05425459).
Other nonpharmacologic approaches to HFpEF management include stimulation-based technologies, nerve ablation therapy and mechanical circulatory support devices, though their utility is less clear and further research into their efficacy is warranted.34,43-45
The Way Ahead
Despite its increasing incidence and prevalence, HFpEF remains a complex and incompletely understood clinical syndrome, though we have recently seen exciting advances in medical management. We look forward to ongoing large-scale clinical trials of both pharmacologic and device-based therapies which are critical in advancing our understanding of this highly prevalent disease.

References
- Bozkurt B, Ahmad T, Alexander K, et al. HF STATS 2024: heart failure epidemiology and outcomes statistics an updated 2024 Report from the Heart Failure Society of America. J Card Fail 2025;31:66-1164.
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591-602.
- Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC Scientific Statement. J Am Coll Cardiol 2023;81:1810-34.
- Larson KF, Malik A, Brozovich FV. Aging and heart failure with preserved ejection fraction. Compr Physiol 2022;12:3813-22.
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9.
- Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013;10:401-10.
- Obokata M, Reddy YNV, Pislaru SV, et al. Evidence Supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6-19.
- Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013;6:279-86.
- Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol 1993;72:310-3.
- Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC Study Community Surveillance. Circulation 2018;138:12-24.
- Brown S, Biswas D, Wu J, et al. Race- and ethnicity-related differences in heart failure with preserved ejection fraction using natural language processing. JACC Adv 2024;3:101064.
- Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021; Mar 1:S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022.
- Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;81:1835-78.
- Desai AS, Lam CSP, McMurray JJV, Redfield MM. How to manage heart failure with preserved ejection fraction: practical guidance for clinicians. JACC Heart Fail 2023;11:619-36.
- Reddy YNV, Carter RE, Obokata M, et al. A Simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861-70.
- Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297-317.
- Pandey A, Shah SJ, Butler J, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1166-87.
- Omote K, Hsu S, Borlaug BA. Hemodynamic assessment in heart failure with preserved ejection fraction. Cardiol Clin 2022;40:459-72.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451-61.
- Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089-98.
- Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail 2019;6:927-35.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary. Circulation 2022;145:e876-e94.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383-92.
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34-42.
- Solomon SD, McMurray JJV, Vaduganathan M, et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2024;391:1475-85.
- Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609-20.
- McMurray JJV, Jackson AM, Lam CSP, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020;141:338-51.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989-1002.
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205-16.
- Kosiborod MN, Abildstrom SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023;389:1069-84.
- Packer M, Zile MR, Kramer CM, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med 2025;392:427-37.
- Borlaug BA, Zile MR, Kramer CM, et al. Effects of tirzepatide on circulatory overload and end-organ damage in heart failure with preserved ejection fraction and obesity: a secondary analysis of the SUMMIT trial. Nat Med 2025;31:544-51.
- Driggin E, Goyal P. Malnutrition and sarcopenia as reasons for caution with GLP-1 receptor agonist use in HFpEF. J Card Fail 2024;30:610-2.
- Granegger M, Gross C, Siemer D, et al. Comparison of device-based therapy options for heart failure with preserved ejection fraction: a simulation study. Sci Rep 2022;12:5761.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66.
- Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935-44.
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991-1001.
- Brugts JJ, Radhoe SP, Clephas PRD, et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. Lancet 2023;401:2113-23.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary. J Am Coll Cardiol 2022;79:1757-80.
- Jagadeesan V, Gray WA, Shah SJ. Atrial shunt therapy for heart failure: an update. J Soc Cardiovasc Angiogr Interv 2023;2:101203.
- Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet 2022;399:1130-40.
- Gustafsson F, Petrie MC, Komtebedde J, et al. 2-Year outcomes of an atrial shunt device in HFpEF/HFmrEF: results from rEDUCE LAP-HF II. JACC Heart Fail 2024;12:1425-38.
- Rosalia L, Ozturk C, Shoar S, et al. Device-based solutions to improve cardiac physiology and hemodynamics in heart failure with preserved ejection fraction. JACC Basic Transl Sci 2021;6:772-95.
- Coats AJS, Abraham WT, Zile MR, et al. Baroreflex activation therapy with the Barostim device in patients with heart failure with reduced ejection fraction: a patient level meta-analysis of randomized controlled trials. Eur J Heart Fail 2022;24:1665-73.
- Burkhoff D, Maurer MS, Joseph SM, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail 2015;3:275-82.
Clinical Topics: Heart Failure and Cardiomyopathies, Acute Heart Failure
Keywords: Cardiology Magazine, ACC Publications, Heart Failure, Preserved Ejection Fraction, Heart Failure

