Cover Story | Clinical Research in a Big Data, High-Tech World

Clinical research has changed dramatically over the last decade and is poised to change even more during the next. And with patient care hanging in the balance and health status falling in many places, improving the efficiency, effectiveness, person-centeredness, inclusivity and integration of the clinical trials enterprise is a priority.
This month, Cardiology talks to experts across the field and explores this bold, new universe of clinical research, where big data, big tech and big ideas all share space with the usual networks of trialists and sponsors.
 Robert W. Yeh, MD, MSc, FACC
Robert W. Yeh, MD, MSc, FACC
 Harriette Van Spall, MD, MPH
Harriette Van Spall, MD, MPH
 Robert Califf, MD, PhD, MACC
Robert Califf, MD, PhD, MACC
The Push of Progress, The Pull of Technology
Building the Big Data Platforms
ACC continues to lead the digital transformation of health care. Collaborations underway through its Innovation programs will not only contribute to transforming cardiovascular practice and improving heart health, but also help with collecting patient data and making sense of it all. From creating a home health monitoring platform, called cliexa-Pulse, to data abstraction with Carta Healthcare, to work to move AI and digital technologies forward through collaborations with GE Healthcare and the Consumer Technology Association, ACC is working towards its vision of innovation and knowledge that optimizes cardiovascular care and outcomes.
Learn more about ACC's Innovation Program and partners at ACC.org/InnovationProgram.
Click here to read an article from Cardiology's archive on how AI and machine learning can push the boundaries of medicine and ACC's collaboration with the Yale-New Haven Hospital's Center for Outcomes Research and Evaluation.
There are likely many reasons the clinical trials world is undergoing such rapid change, but to Robert W. Yeh, MD, MSc, FACC, director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at the Beth Israel Deaconess Medical Center (BIDMC) and endowed chair in cardiovascular outcomes research at Harvard Medical School, much can be explained by what he calls the push of progress and the pull of technology.
"In large part because of the traditional randomized controlled trials (RCTs) done over the last 50 years – the TIMI and GUSTO trials and many others – we've gotten so much better at preventing and treating cardiovascular disease that it has gotten harder and harder to conduct clinical trials of new interventions just because we've changed the natural history of the disease. The expected event rates are so low," he says.
An interventional cardiologist and an expert in the use of nonrandomized data for clinical decision-making, Yeh also notes that a trial of a new medication 20 years ago might have required 10,000 patients to be adequately powered, but now it requires 20,000 patients. Citing PCSK9 inhibitors as a good example, Yeh says it's become even harder to demonstrate that new therapies can save lives. Large trials were conducted and showed a reduction in myocardial infarction, but no differences in cardiovascular mortality, he says. "This makes us wonder whether the events we're preventing are as important as they once were. And this makes it harder and more expensive to show benefit for new therapeutics using traditional methods."
Paired with this incentive to conduct more efficient clinical research is the pull – or the enabling factor – of technology. "Electronic health records (EHRs) have greater functionality," says Yeh.
"We're able to collect information from patients directly with sensors, wearables and mobile technology. And we can recruit patients in more creative ways using online platforms, like it was done for the ADAPTABLE trial. All of this facilitates more efficient clinical trials, so it's not a surprise that we're moving in that direction," says Yeh.
Robert Califf, MD, PhD, MACC, takes it even further. "Collecting data by a patient going to a clinic visit with a study coordinator allows for a lot of consistency and rigor, but it's a very limited slice of human existence. When you start monitoring research participants at home, around the clock, with sensors and a sleep monitor and running a wide array of tests on them, well, that's a whole different view of the human condition."
Califf certainly knows the ins and outs of the clinical trial enterprise. He was a founding director of the Duke Clinical Research Institute, a director of the Duke Translational Medicine Institute, and a Deputy Commissioner (2015-2016) and then Commissioner of the U.S. Food & Drug Administration (FDA; 2016-2017). He is now the head of Clinical Policy and Strategy at Verily Life Sciences and Google Health.
Efficacy vs. Effectiveness, But Still Randomized
Diversity in Clinical Trials
Lack of diversity in clinical trials has been called a "moral, scientific, and medical issue."1 After all, if clinical trials provide the critical evidence base for evaluating the safety and efficacy of new medicines and medical products, how can we presume to have done that if the trial enrolled a nonrepresentational proportion of, say, women and members of different racial and ethnic groups?
For many in the field, the first step to improving diversity in clinical trial populations is to diversify trial leadership.
The gender inequality in leadership is stark in cardiology: Although trials led by women investigators do a better job of enrolling participants who are female and underrepresented groups, less than half of cardiovascular trials published in three high-impact factor journals between 2014 and 2018 had women on their executive committees and less than 10% of publications from these high-impact, multicenter trials had a woman in the lead author position.2
"This gap, underpinned by systemic sexism, has not been adequately addressed in the research enterprise," writes Harriette Van Spall, MD, MPH, and a group of colleagues representing the Global CardioVascular Clinical Trialists (CVCT) Forum and Women as One Scientific Expert Panel in a recent Journal of the American College of Cardiology paper.
The gap is even larger in procedural subspecialties, amplifying the marked gender gap in these subspecialties. However, even in heart failure, where the proportion of women clinicians is higher than in other cardiovascular subspecialties, there is a persistent and unchanging lack of diversity in trial leadership.
The factors behind these disparities are many but it essentially boils down to a system that sees women holding supporting roles but few leadership roles. And many women opting out of careers focused on clinical research, Van Spall relates in an interview.
"We need to be more thoughtful about inclusivity and thinking proactively about women who are qualified co-investigators and collaborators, so we stop seeing what we see often now, which is a group of male researchers in leadership with a woman statistician," says Van Spall.
"It's not about making diversity a check box item, but about marrying all the things that are important to us – capability and excellence, as well as diversity in gender, minority and geographic representation – to assure we're leveling the playing field for everyone and generating high-quality, nonbiased evidence," she adds.
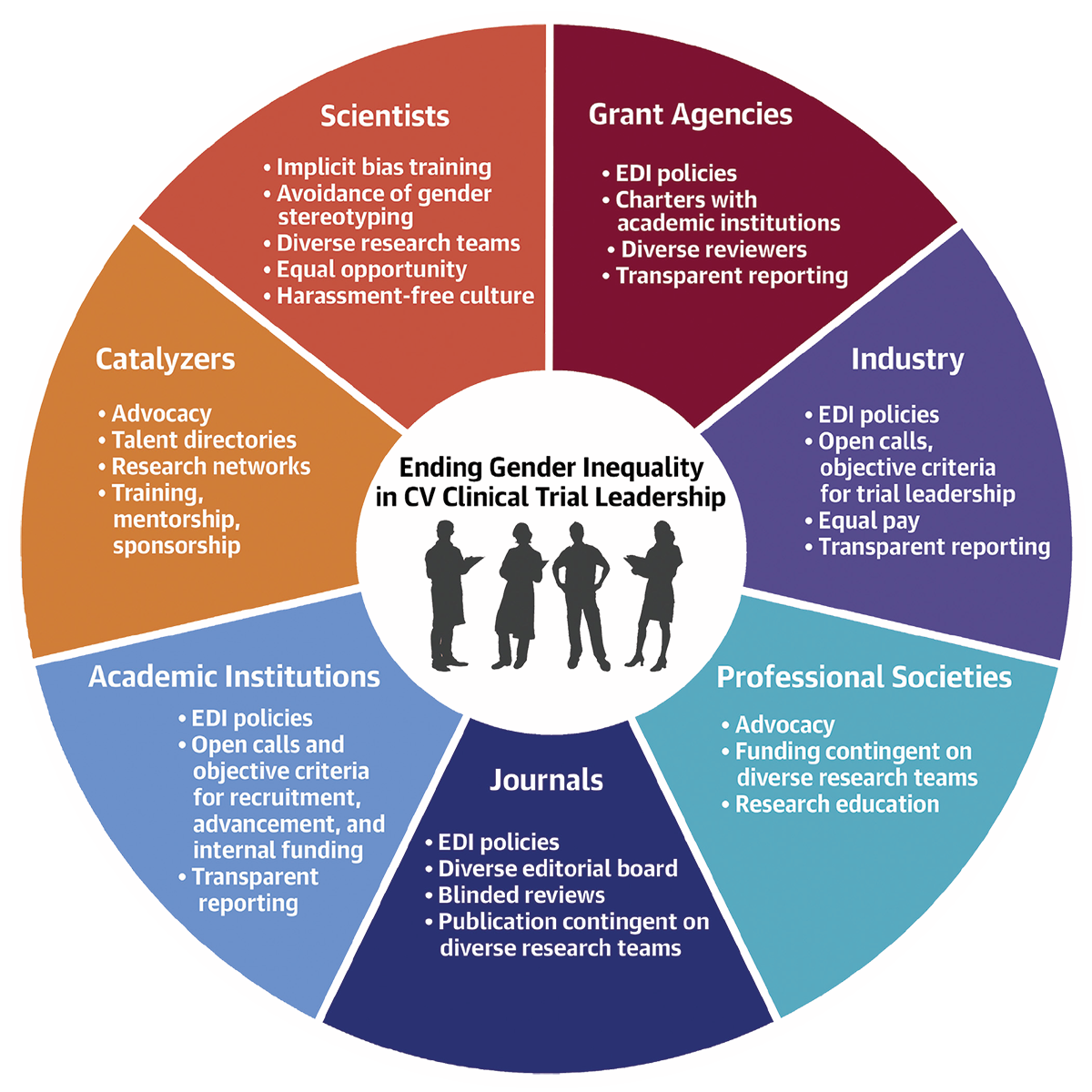
Van Spall and colleagues make a range of suggestions for improving gender balance in clinical trial leadership, but stress that only deliberate action and monitoring by all stakeholders – individuals, institutions, industry, funders, journals – will effect real change (Figure).
References
- Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol 2019;44:148-72.
- Van SHGC, Lala A, Deering TF, et al. Ending gender inequality in cardiovascular clinical trial leadership. J Am Coll Cardiol 2021;77:2960-72.
To be clear, clinical trials have revolutionized clinical care over the last 50 years and the RCT remains the gold standard for evaluating new treatments and comparing treatments. But with this, traditional RCTs have several well-known limitations and they are costly, making them important contributors to drug-development costs. These limitations have led to an imperative to tweak the methodology of RCTs.
"I would still say that nothing beats random allocation because it's just really hard to know what impacted the outcome if there wasn't randomization," says Harriette Van Spall, MD, MPH, a cardiologist and scientist in the Knowledge Translation and Heart Failure programs at the Population Health Research Institute at McMaster University. She is one of the leaders in the field of pragmatic trials.
"Randomized controlled trials can range from highly pragmatic to highly explanatory. Pragmatic trials evaluate the effectiveness of an intervention in routine clinical practice conditions, which differs from explanatory clinical trials, which are designed to evaluate the efficacy of an intervention under optimal conditions," says Van Spall.
In an explanatory trial, the estimate of the intervention effect is usually maximized by selecting patients most likely to respond and comparing efficacy (usually to placebo) under ideal, experimental conditions. Pragmatic clinical trials, on the other hand, blend the benefits of randomization with routine, everyday clinical practice.
"In pragmatic trials, while hard outcomes are important, we also really want to consider what's important to the patient, particularly as disease progresses. We know not all patients choose the hard endpoint as their priority. Some prefer greater quality of life, more integrated care with fewer subspecialists involved, all kinds of things that may not be captured when you're just looking at major adverse cardiovascular event rates," says Van Spall.
And we need to know about these patient priorities as they happen in real-world settings, not conducted in a research clinic with trial procedures that are not congruent with clinical practice.
"The price you pay in a pragmatic trial is that, by the very nature of the trial, your estimated treatment effect will be lower than in an explanatory trial," she adds. "The benefit is that you're testing an intervention in a setting that mirrors everyday clinical care and among typical patients suffering from the disease. So, you have high external validity, meaning you can apply these results to a broad spectrum of patients, knowing what the treatment effect and adverse effects are in these patients."
The ADAPTABLE trial, of course, comes to mind. The results, presented at ACC.21 and simultaneously published in the New England Journal of Medicine, showed that in patients with established atherosclerotic cardiovascular disease, there were no significant differences in cardiovascular events or major bleeding between patients assigned to 81 mg aspirin compared with 325 mg aspirin daily.1 However, the researchers saw a clear preference for the lower dose among patients, and the rate of dose switching in the 325 mg arm was significantly higher than in the 81 mg arm (41.6% vs. 7.1%).
"I think the most important thing about ADAPTABLE is that we've shown for the big outcomes studies that we can now use EHRs and interact with people directly using their cell phones. All at a cost that is radically lower than a traditional RCT. But this doesn't mean we've taken care of all the methodologic issues that exist with clinical trials in general, like the crossover rate," says Califf.
Is RWE Fit For Use?
The FDA has long used real-world data (RWD) and real-world evidence (RWE) to monitor postmarket safety and adverse events and to make regulatory decisions. As defined by the FDA, RWD are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. RWE is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD. Put otherwise, RWD are raw information that, after analysis, become RWE.
The 21st Century Cures Act, passed in 2016 during Califf's time as FDA commissioner, places additional focus on the use of these types of data to support regulatory decision-making, including the approval of new indications for approved drugs. The FDA has written a draft for their RWE program and efforts are ongoing to issue comprehensive guidance for industry (by December 2021).
If one seeks to use nonrandomized RWE, otherwise known as observational treatment comparisons, the observational study essentially needs to duplicate many of the circumstances of an RCT. This has been done several times with good success.2
In a recent example, researchers, led by Yeh, linked a group of Dual Antiplatelet Therapy (DAPT) study patients to the ACC's NCDR CathPCI Registry and compared data elements for the same patients. Of 8,864 DAPT patients, 5,743 (65%) were successfully matched to data in the CathPCI Registry.
There was strong agreement with many data elements, including demographics and procedural characteristics, modest agreement for some prior history and risk factors, and poor agreement for clinical presentation. According to the researchers, most notably, angina was more likely to be classified as unstable in the CathPCI Registry vs. the DAPT Study.
The results suggest that CathPCI Registry data could be used to support future clinical trials (as was done in the SAFE-PCI study3), decreasing the burden of data collection on sites participating in both trials and the registry. However, variables that could be considered more subjective, such as clinical presentation, would likely need to be defined more precisely, write Yeh and colleagues.
"Whether these findings generalize to other types of data in the CathPCI Registry or other clinical registries is unknown and remains a rich area for future inquiry," Yeh says. "Overall, however, the data are promising in pointing to an important mechanism to make clinical trials more feasible and less costly, helping to overcome one of the most significant barriers to clinical research."
RCT DUPLICATE
Transforming the Faces of Clinical Trials

"If clinical research is to benefit all of humanity, the trials upon which drug/device approval are based must be representative of the whole population, but we are far from that ideal," says Quinn Capers IV, MD, FACC, chair of ACC's Diversity and Inclusion Committee.
Three pieces of data underscore the current state of affairs, he states: 1) 91% of patients in a national registry of TAVR procedures were White; 2) 91% of patients in contemporary coronary stent trials were White, and 3) while Blacks comprise 13% of the U.S. population, they tend to be 5% or less of cardiovascular trial participants. The lack of diversity in clinical trial populations deprives minority and women patients from the potentially lifesaving benefits of participating in trials of cutting-edge therapies.
"A lack of diversity and cultural competency among principal investigators and research coordinators have been proposed as culprits," he adds. "Minority communities may also have a lingering mistrust regarding human research and experimentation. This mistrust is not misguided but based on historical atrocities committed against vulnerable and disadvantaged communities in the name of biomedical research."
"All these culprits can be overcome by active engagement with minority communities with trusted messengers, recruiting diverse individuals to serve as cardiovascular physician-scientists and research coordinators, and training research teams in cultural humility and bias mitigation. The Diversity and Inclusion Committee is committed to supporting all these activities and more," says Capers.
The RCT DUPLICATE project is a landmark study designed to perform structured assessments of 30 nonrandomized observational cohorts, matching them to 30 RCTs on the same topic. The project launched in late 2017 to determine whether the use of RWE would have led to the same regulatory decisions.
Initial findings, published online in December 2020 in Circulation, shared the results of 10 of the RWE studies.4 The researchers, led by Jessica Franklin, PhD, and Sebastian Schneeweiss, MD, ScD, from Brigham and Women's Hospital in Boston, showed that despite attempts to emulate the RCT design in RWE populations, differences remained. Notably, regulatory decisions were equivalent in only six of 10 trials matched, meaning they reached similar conclusions with respect to assessment of traditional statistical significance as in the RCT. Three of the four trials that did not reach regulatory agreement were placebo-controlled trials, which highlights the challenges of emulating these types of trials in RWE.
The RWE emulations achieved a hazard ratio estimate that was within the 95% confidence interval from the corresponding RCT in eight of 10 studies and, in nine of 10, either the regulatory or estimate agreement success criteria were fulfilled.
"What we don't mean to imply is that all real-world evidence needs to calibrate against an RCT. That would be totally defeating the purpose of real-world evidence because we want to go beyond the randomized evidence, to expand to different populations and different endpoints that you don't study with RCTs," Schneeweis said at a recent event sponsored by the National Institutes of Health.
Nonrandomized Data For Causal Inference
How about designing observational studies that allow for causal inference? It's possible, but it's important to recognize these are sophisticated observational studies that differ from traditional observational studies in important ways.
"We've worked a lot with the FDA on this issue because they want to use RWD, but they want to understand what can and cannot be interpreted as causal," says Yeh. "One has to have a really good understanding of the statistical methodology, along with the clinical subject area to understand whether or not an observational method, and which method, is appropriate for the particular question," he adds. The investigators at the BIDMC Smith Center include active clinicians who also have graduate training in epidemiologic methods as well as a PhD causal inference expert from the Harvard School of Public Health. This interdisciplinary group works closely together to make sure they're asking the right questions and answering them in a rigorous fashion.
A recent example is the SAFE-PAD trial, presented at ACC.21 and published concurrently in JAMA Internal Medicine.5 The FDA asked the BIDMC Smith Center team, including lead author Eric A. Secemsky, MD, MSc, FACC, to design a real-world study to test whether paclitaxel-coated stents and balloons might be causing an increased risk of death in patients treated for femoropopliteal peripheral artery disease. The concern arose after a late 2018 meta-analysis of RCTs found a late mortality increase starting two to three years after treatment with paclitaxel-coated endovascular devices compared with plain balloons and bare-metal stents.6
Their retrospective cohort analysis of Medicare beneficiaries prespecified both a quasi-experimental (instrumental variable) design and inverse probability weighting as the analytic methods for the endpoints to correct for potential confounding owing to imbalances in observed characteristics. Other statistical techniques and sensitivity analyses were used to establish greater confidence in the causal interpretation of the study.
They found that no matter which way they looked at the data, they were unable to detect any signal of harm for paclitaxel-coated devices.
"I'm sure the sophistication of the methods was lost on some and this was seen as just another nonrandomized claims data study. But the FDA requested we do a study to evaluate the causal impact of these devices. That's what this was," says Yeh.
Project Baseline: Mapping Human Health
The Project Baseline Health Study is seeking to harness the world of big data and cloud computing and understand "the dynamic interplay of the biological, behavioral, environmental and social systems and the impact of time that underlie health status."7 Fully funded by Verily Inc., an Alphabet company (along with Google), Baseline has the deep pockets and the technical know-how to do it. They are developing tools to enable the collection and curation of a trove of data unlike anything seen before in the clinical trial space.
"Before cloud computing, we were limited in how we could collect data and how much data we could collect and process. What we've tried to do in Baseline is to create a set of technologies that could absorb, curate and organize multidimensional data about people – some of it continuously collected – involving people as participants rather than subjects," says Califf.
"An extension of that effort is the Baseline platform that we hope can be useful for essentially all types of clinical research from classical epidemiologic studies all the way to the modern version of what you would call a traditional RCT," he adds.
The study has enrolled 2,500 individuals in whom a large volume of multimodality data has been collecting. They are called the "deeply phenotyped cohort."
With four years of data on board, Baseline is starting to present and publish findings. One study that's in press looks at the PHQ9, a standard screening survey for depression, and its relationship to socioeconomic status (SES). A correlation between the two has been made before, but not with the broad range of data available in Baseline, which includes clinical assessments, genetic and metabolomic profiles, multiple measures of proteins, and continuous self-reported and sensor-based monitoring.
"We think the depth of the relationship between PHQ9 and SES, since we've measured so many other things, is really telling us that when we think about intervening on depression, we'd better understand a person's SES. And when we intervene in neighborhoods, for example, to try to improve SES, we need to be aware that a lot of these people are depressed, because that strongly affects motivation and self-efficacy," says Califf.
The dream for Baseline, adds Califf, is that it will one day be a "broad collaboration involving millions of people who are sharing their information to improve knowledge and then participating in clinical trials and registries and observational studies, as indicated, to answer the questions that we're interested in." One thing that's guaranteed is that it will never look like your grandad's RCT.
Upping the Game
ACC Clinical Trials Research: Upping Your Game
The ACC's Clinical Trials Research: Upping Your Game program is one such effort already underway to increase the number of individuals historically underrepresented in cardiology who lead clinical trials, bringing a greater diversity in thinking and perspective to the field and ensuring those working in clinical trial research better reflect all patients with cardiovascular disease.
Women as One
Women as One is an organization founded in 2019 by Roxana Mehran, MD, FACC, and Marie Claude Morice, MD, FACC, to promote talent in medicine by providing unique professional opportunities to female physicians. Click here to read the Cardiology feature on this initiative.
This article was authored by Debra L. Beck, MSc.
References
- Jones WS, Mulder H, Wruck LM, et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N Engl J Med 2021;384:1981-90.
- Butala NM, Faridi KF, Secemsky EA, et al. Comparing baseline data from registries with trials. JACC Cardiovasc Interv 2021;14:1386-8.
- Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv 2014;7:857-67.
- Franklin JM, Patorno E, Desai RJ, et al. Emulating randomized clinical trials with nonrandomized real-world evidence studies. Circulation 2021;143:1002-13.
- Secemsky EA, Shen C, Schermerhorn M, Yeh RW. Longitudinal assessment of safety of femoropopliteal endovascular treatment with paclitaxel-coated devices among medicare beneficiaries: The SAFE-PAD Study. JAMA Intern Med 2021;May 16:[Epub ahead of print].
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245.
- Arges K, Assimes T, Bajaj V, et al. The Project Baseline Health Study: a step towards a broader mission to map human health. Npj Digit Med 2020;3(1):84.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Atherosclerotic Disease (CAD/PAD), Aortic Surgery, Cardiac Surgery and Heart Failure, Lipid Metabolism, Novel Agents, Acute Heart Failure, Interventions and Vascular Medicine, Sleep Apnea
Keywords: ACC Publications, Cardiology Magazine, Academies and Institutes, Accidental Falls, African Americans, Ambulatory Care, Arm, Artificial Intelligence, Aspirin, Biological Science Disciplines, Biomedical Research, Biometry, Boston, Capparis, Cardiology, Cardiovascular Diseases, Cardiovascular System, Causality, Cell Phone, Cohort Studies, Confidence Intervals, Cultural Competency, Data Collection, Decision Making, Delivery of Health Care, Delivery of Health Care, Integrated, Depression, Device Approval, Electronic Health Records, Ethnic Groups, Furunculosis, Global Health, Health Status, Heart Failure, Hospitals, Journal Impact Factor, Leadership, Medicare, Medicine, Morals, Motivation, Myocardial Infarction, National Institutes of Health (U.S.), Outcome Assessment, Health Care, Paclitaxel, Patient Selection, PCSK9 protein, human, Percutaneous Coronary Intervention, Peripheral Arterial Disease, Pharmaceutical Preparations, Policy, Probability, Proprotein Convertase 9, Prospective Studies, Public Health, Quality of Life, Random Allocation, Reference Standards, Registries, Research Design, Research Personnel, Retrospective Studies, Risk Factors, Schools, Schools, Medical, Self Efficacy, Self Report, Sex Factors, Sexism, Sleep, Social Class, Stents, Technology, Transcatheter Aortic Valve Replacement, Translational Medical Research, Universities, Violence, Vulnerable Populations, Innovation
< Back to Listings