Spotlight Series | Microvascular Dysfunction: Coronary Microvascular Disease in Patients With Obstructive CAD
Abnormalities in the coronary circulation are not limited to the epicardial arteries and include disease of the distal vessel and the microcirculation.1 Coronary microvascular dysfunction (CMD) is used to describe this spectrum of structural and functional abnormalities in the coronary microcirculation, leading to impaired coronary blood flow resulting in myocardial ischemia and angina.
CMD is increasingly recognized across several cardiovascular diseases including ischemia with nonobstructive coronary artery disease (INOCA), myocardial infarction without obstructive CAD (MINOCA), heart failure with preserved ejection fraction, and Takotsubo syndrome. However, it is also prevalent in patients with epicardial coronary disease including those with an MI and refractory angina despite successful PCI.1
Atherosclerosis, which may long precede the development of obstructive coronary artery disease (CAD), affects endothelial and vasomotor activity of the proximal and distal coronary artery circulation by the chronic release of multiple vasoactive and atherogenic molecules.2 This in turn results in CMD characterized by endothelial- and nonendothelial-dependent microvascular dysfunction and coronary vasomotor dysfunction.
Therefore, it is not surprising that CMD coexists in patients with obstructive CAD. In acute MI, the mechanisms involved in the pathogenesis of microvascular obstruction include the preexistence of CMD from atherosclerosis and a series of pathological changes that collectively lead to obstruction of the microvessels.3
Stable Obstructive CAD
CMD is one of the underlying pathophysiologic mechanisms of ongoing angina in patients with obstructive CAD despite complete revascularization with PCI or CABG. In patients with underlying CMD, revascularization increases blood flow to an already abnormal circulation. Chronic adaptation of the coronary microcirculation to a low perfusion pressure distally to a stenosis may cause adverse cardiovascular remodeling and inability to achieve maximal dilatation that results in delayed recovery of coronary resistive vessel function after restoration of normal basal coronary blood flow with coronary angioplasty.4
A study by Ong, et al., reported evidence of microvascular and epicardial vasoconstriction detected by acetylcholine provocative testing in 49% of patients undergoing coronary angiography for recurrent angina after PCI despite patent coronary arteries.5
Discrepancies in severity of myocardial ischemia on stress testing and the severity of epicardial coronary disease by invasive angiography may be in part due to CMD.
Small Vessel Impact on Angina, Ischemia

The diagnosis of CAD has focused on presence of obstructive epicardial CAD. It is estimated that at least two of five male and female patients with angina referred for elective coronary angiography have nonobstructive epicardial coronary arteries, with rates relatively higher in women.
Missed the first two articles in this four-part series, developed by invited Guest Editor, C. Noel Bairey Merz, MD, FACC? Click here to learn about the invasive and noninvasive diagnosis of small vessel disease and about the limitations of PCI.
CMD affects the physiologic assessment of epicardial stenosis. In patients with obstructive CAD, coexisting CMD may result in evidence of ischemia in regions supplied by arteries without stenosis and can magnify ischemia in regions with obstructive CAD.6 Fractional flow reserve (FFR) values for physiologic assessment of epicardial stenosis may be pseudonormal in the presence of CMD and therefore underestimate the physiologic severity of stenosis.7
Van de Hoef reported that up to one-third of patients with obstructive CAD and normal FFR have concomitant CMD by coronary flow velocity reserve (CFVR).8 CFR assessment to determine the hemodynamic effects of stenosis and CMD in patients with obstructive CAD should be considered to determine if CMD may be contributing to the normal FFR particularly in cases with evidence of myocardial ischemia.
Residual angina post revascularization is a common clinical problem. CMD may account for the high frequency of angina post-randomization in clinical trials of revascularization in stable ischemic heart disease (Figure 1).9-14 For example, in the COURAGE trial among 2,287 patients with stable significant CAD with objective evidence of ischemia at the five-year follow-up, 26% of patients in the PCI group and 28% of patients in the medial-therapy group had residual angina.10
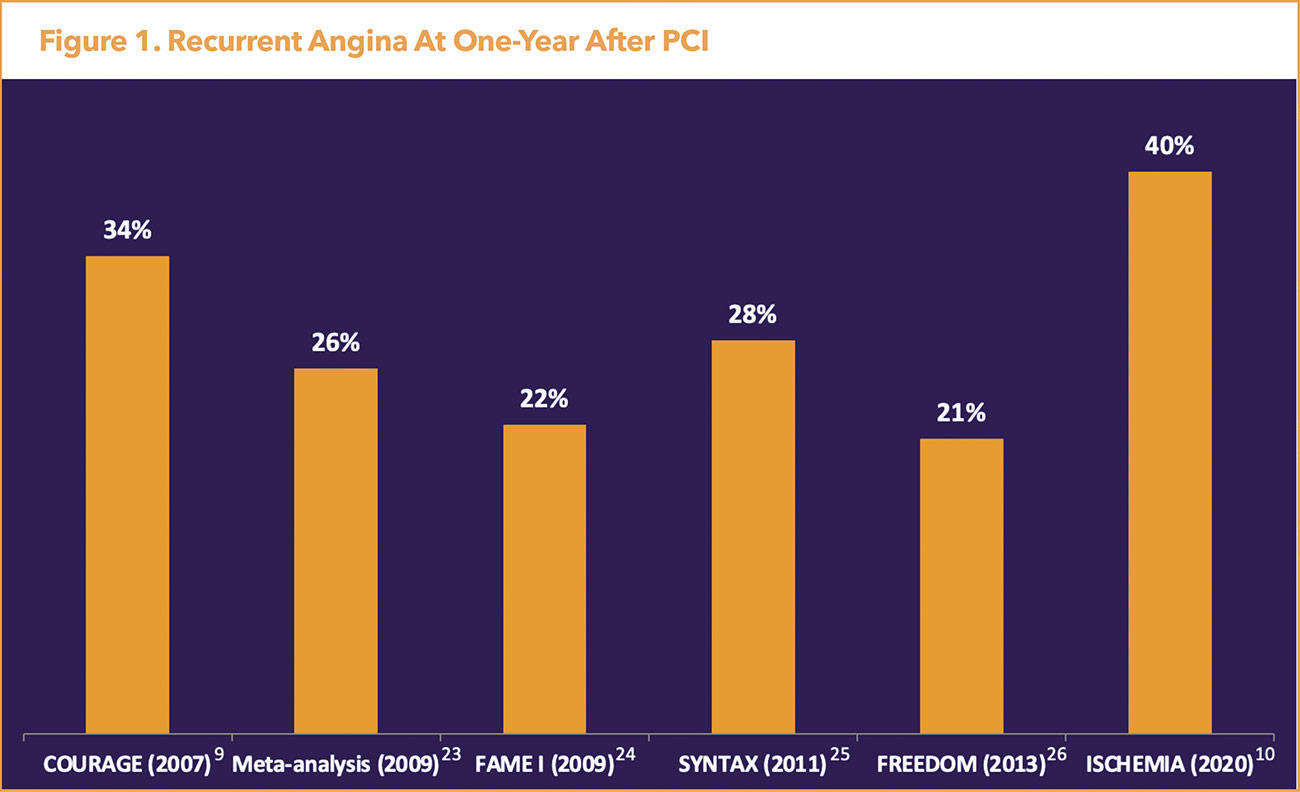
Additional revascularization occurred in 21.1% of patients in the PCI group and 32.6% of those in the medical-therapy group for angina that was unresponsive to maximal medical therapy or evidence of worsening ischemia on noninvasive testing.10 More recently, the ISCHEMIA trial evaluated outcomes in PCI compared with medical therapy among 5,179 patients with high ischemic burden (moderate or severe ischemia on stress testing) and stable CAD.13
Among patients with a baseline Seattle Angina Questionnaire Angina Frequency score of 50 (weekly angina), only 45% of those treated invasively and 15% of those treated conservatively were angina-free at three months.15 Of note, the ISCHEMIA trial excluded patients who had unacceptable angina despite maximal medical therapy, resulting in less symptomatic patients. In fact, 35% of participants did not have angina at baseline.

Further, in ISCHEMIA patients were diagnosed with ischemia prior to angiography and 21% were found to have no obstructive CAD on coronary computed tomography angiography consistent with an INOCA diagnosis.16 This illustrates the interaction between epicardial CAD and CMD. Lastly, COURAGE and ISCHEMIA demonstrated no significant reduction in major adverse cardiovascular events (MACE) or mortality in patients in the treatment arm who received PCI.10,13 The lack of benefit after revascularization emphasizes the importance of investigating the microcirculation and assessing for CMD.
The presence of CMD has important clinical and prognostic implication in patients with obstructive CAD. Patients who had revascularization deferred on the basis of a normal FFR but had abnormal CFVR had increased risk of MACE at 10 years.8 Whereas, an abnormal FFR (<0.75) but normal CFVR was associated with equivalent clinical outcomes as compared with cases where both FFR and CVFR were normal.
In patients undergoing elective PCI, an intracoronary Doppler-derived CFR <2.5 after PCI predicted recurrence of angina or ischemia within one-month.17 Further, Global CFR measured by positron emission tomography has been shown to modify the effect of revascularization; whereby, only patients with severely reduced CFR were shown to have a benefit from complete revascularization with CABG.6 Global CFR was also associated with adverse cardiovascular events independently of luminal angiographic severity.6
A meta-analysis of 79 studies and 59,740 individuals using multiple modalities of CFR measurements demonstrated reduced CFR was associated with 3.45-fold higher risk of MACE and 3.78-fold higher risk of mortality.18
Acute Myocardial Infarction

Despite the high success rate of coronary reperfusion with primary PCI, impaired myocardial reperfusion due to microvascular obstruction occurs in approximately 50% of patients following STEMI.19 Microvascular obstruction associated with CMD is thought to be multifactorial including endothelial dysfunction, reperfusion injury, embolization of the thrombus distally, and intramyocardial edema and hemorrhage.
Studies have demonstrated that CMD measured by reduced invasive CFR and elevated index of microvascular resistance (IMR) or hyperemic microvascular resistance (HMR) following STEMI are associated with adverse clinical outcomes.20 Microvascular obstruction measured by cardiac magnetic resonance imaging has been correlated with an IMR >40.21 Further, microvascular obstruction is associated with adverse left ventricular remodeling and death.19 A recent meta-analysis in STEMI patients demonstrated prognostic significance of severe CMD; whereby, IMR >40 or HMR >3 mm Hg/cm/sec was associated with a 3.42-fold higher risk of MACE (pooled hazard ratio, 3.42; confidence interval, 2.45-4.79).22
Management of Obstructive CAD Beyond Revascularization

Aggressive risk factor modification is essential with smoking cessation and optimal control of lipids, blood pressure and diabetes. Guideline-directed optimal medical therapy (OMT), including antiplatelet therapy with aspirin and P2Y12 inhibitor, lipid-lowering agents, and antianginals with beta-blockers, dihydropyridine calcium-channel blockers and long-acting nitrates, should be optimized. Guideline-direct therapeutic lifestyle change (TLC) regarding referral to cardiac rehabilitation, exercise, nutrition and stress management are cornerstones of the therapeutic approach. Other agents including ivabradine, ranolazine and L-arginine may be indicated in patients with refractory angina.
Beyond medication optimization, there are noninvasive therapies available including enhanced external counter-pulsation (EECP) for patients with angina refractory to medical therapy and revascularization. EECP is approved for patients with CCS III-IV angina. It augments coronary blood flow using pneumatic cuffs around the lower extremities that inflate during diastole and deflate in systole to decrease afterload and increase venous return.
EECP in a randomized sham-controlled trial of patients with CAD and positive exercise treadmill test, reduced angina and time to exercise-induced ischemia.23 Also, Luo, al., demonstrated that EECP also has beneficial effects for patients without obstructive CAD with CMD.24 EECP has also been shown to improve blood pressure and glycemic control.25
Mechanisms by which EECP improves angina include release of vasoactive factors including nitric oxide, vascular endothelial growth factor, and fibroblast growth factor, as well as circulating CD34+ progenitor stem cells that improve endothelial function, promote collateralization, enhance ventricular function, improve oxygen consumption, result in regression of atherosclerosis and peripheral training effects similar to exercise.26
Conclusions
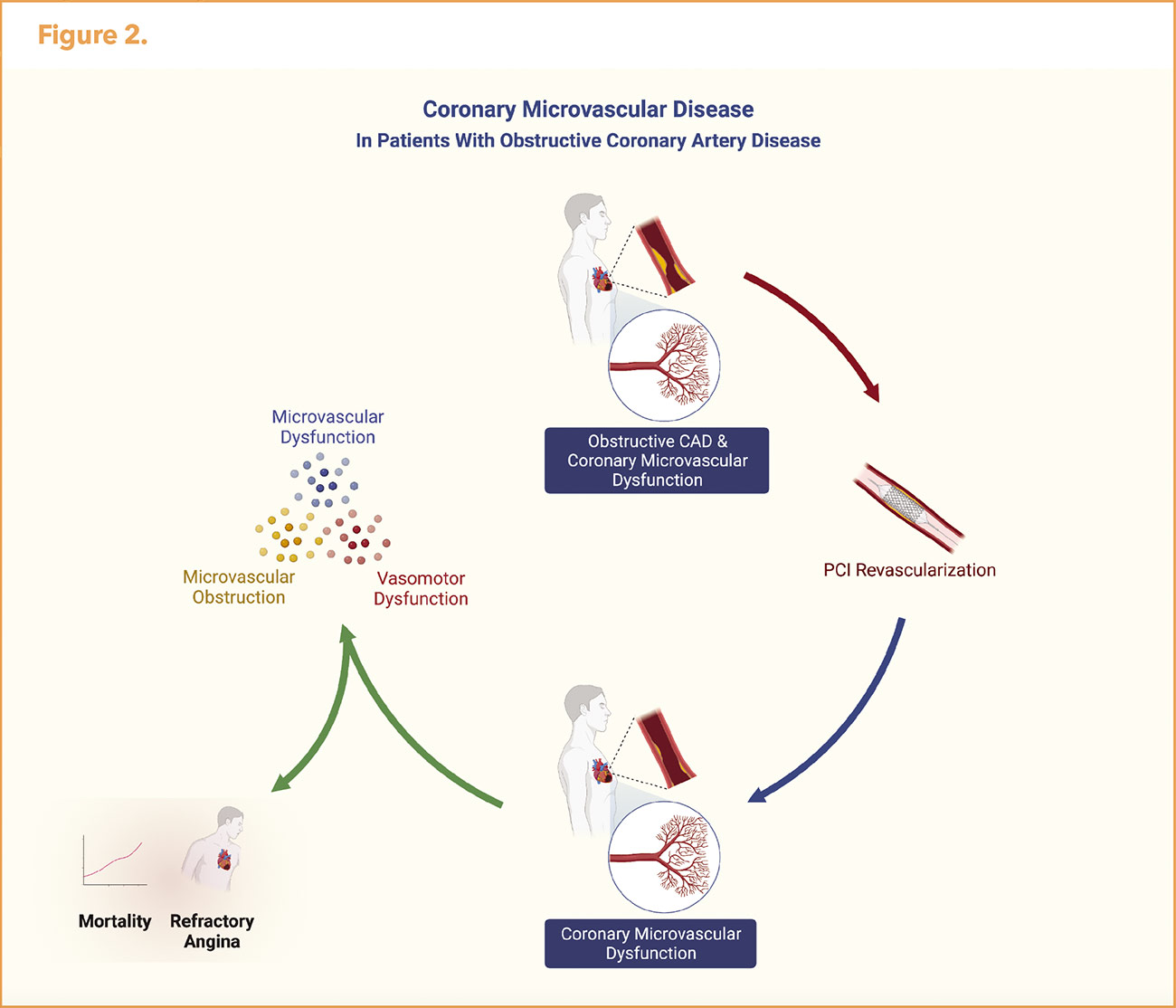
CMD and microvascular obstruction are common in stable and acute ischemic heart disease. CAD affects vasomotor activity of the distal coronary artery circulation resulting in CMD and chronic myocardial ischemia. The impact of CMD on angina and prognosis underscores the need to optimize medical therapy to treat residual CMD that may not be improved with revascularization (Figure 2).


This article was authored by Odayme Quesada, MD, FACC, from The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital, and the Women's Heart Center at the Christ Hospital Heart and Vascular Institute; and Timothy D. Henry, MD, FACC, from The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital, both in Cincinnati, OH.
References
- Del Buono MG, Montone RA, Camilli M, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:1352-71.
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109:III27-32.
- Bulluck H, Foin N, Tan JW, et al. Invasive assessment of the coronary microcirculation in reperfused ST-segment-elevation myocardial infarction patients: Where do we stand? Circ Cardiovasc Interv 2017;10.
- Uren NG, Crake T, Lefroy DC, et al. Delayed recovery of coronary resistive vessel function after coronary angioplasty. J Am Coll Cardiol 1993;21:612-21.
- Ong P, Athanasiadis A, Perne A, et al. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in-stent restenosis. Clin Res Cardiol 2014;103:11-9.
- Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19-27.
- Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639-53.
- van de Hoef TP, van Lavieren MA, Damman P, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 2014;7:301-11.
- Abdallah MS, Wang K, Magnuson EA, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA 2013;310:1581-90.
- Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16.
- Cohen DJ, Van Hout B, Serruys PW, et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med 2011;364:1016-26.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009;373:1190-7.
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395-1407.
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24.
- Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med 2020;382:1408-19.
- Reynolds HR, Picard MH, Spertus JA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: The CIAO-ISCHEMIA Study. Circulation 2021;144:1008-1023.
- Serruys PW, di Mario C, Piek J, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation 1997;96:3369-77.
- Kelshiker MA, Seligman H, Howard JP, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J 2022;43:1582-93.
- van Kranenburg M, Magro M, Thiele H, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 2014;7:930-9.
- Maznyczka AM, Oldroyd KG, Greenwood JP, et al. Comparative significance of invasive measures of microvascular injury in acute myocardial infarction. Circ Cardiovasc Interv 2020;13:e008505.
- Bulluck H, Foin N, Cabrera-Fuentes HA, et al. Index of microvascular resistance and microvascular obstruction in patients with acute myocardial infarction. JACC Cardiovasc Interv 2016;9:2172-2174.
- Canu M, Khouri C, Marliere S, et al. Prognostic significance of severe coronary microvascular dysfunction post-PCI in patients with STEMI: A systematic review and meta-analysis. PLoS One 2022;17:e0268330.
- Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol 1999;33:1833-40.
- Luo C, Liu D, Wu G, et al. Effect of enhanced external counterpulsation on coronary slow flow and its relation with endothelial function and inflammation: a mid-term follow-up study. Cardiology 2012;122:260-8.
- Campbell AR, Satran D, Zenovich AG, et al. Enhanced external counterpulsation improves systolic blood pressure in patients with refractory angina. Am Heart J 2008;156:1217-22.
- Michaels AD, McCullough PA, Soran OZ, et al. Primer: practical approach to the selection of patients for and application of EECP. Nat Clin Pract Cardiovasc Med 2006;3:623-32.
Clinical Topics: Acute Coronary Syndromes, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Stable Ischemic Heart Disease, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Cardiac Surgery and Heart Failure, Cardiac Surgery and SIHD, Acute Heart Failure, Interventions and ACS, Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Chronic Angina
Keywords: ACC Publications, Cardiology Magazine, Cardiology Magazine Spotlight Series, Acute Coronary Syndrome, Aneurysm, Angiography, Heart Failure, Coronary Artery Disease, Myocardial Revascularization, Angina, Stable, Angina, Unstable, Fractional Flow Reserve, Myocardial
< Back to Listings


