Spotlight Series | Microvascular Dysfunction: Invasive and Noninvasive Diagnosis of Small Vessel Disease

Ischemia with no obstructive coronary arteries (INOCA) refers to myocardial ischemia with stable or unstable anginal symptoms in the setting of normal or nonobstructive coronary arteries.1 Coronary microvascular dysfunction (CMD) and epicardial coronary artery spasm are pathophysiologic mechanisms of INOCA and are diagnosed in up to four in five patients undergoing invasive evaluation for suspected INOCA.2-6
Patients with INOCA are often misdiagnosed as having symptoms of noncardiac origin, as traditional stress tests have low sensitivity for diagnosing CMD7 and diagnosis of vasospasm often requires acetylcholine provocation.8 As CMD and epicardial spasm are associated with adverse long-term prognosis in patients without obstructive coronary artery disease (CAD),9-15 the diagnosis of these INOCA endotypes should be considered in patients with anginal symptoms. Furthermore, stratified medical therapy based on diagnosis of microvascular and/or vasospastic angina has demonstrated improved angina and quality of life in patients with INOCA.1
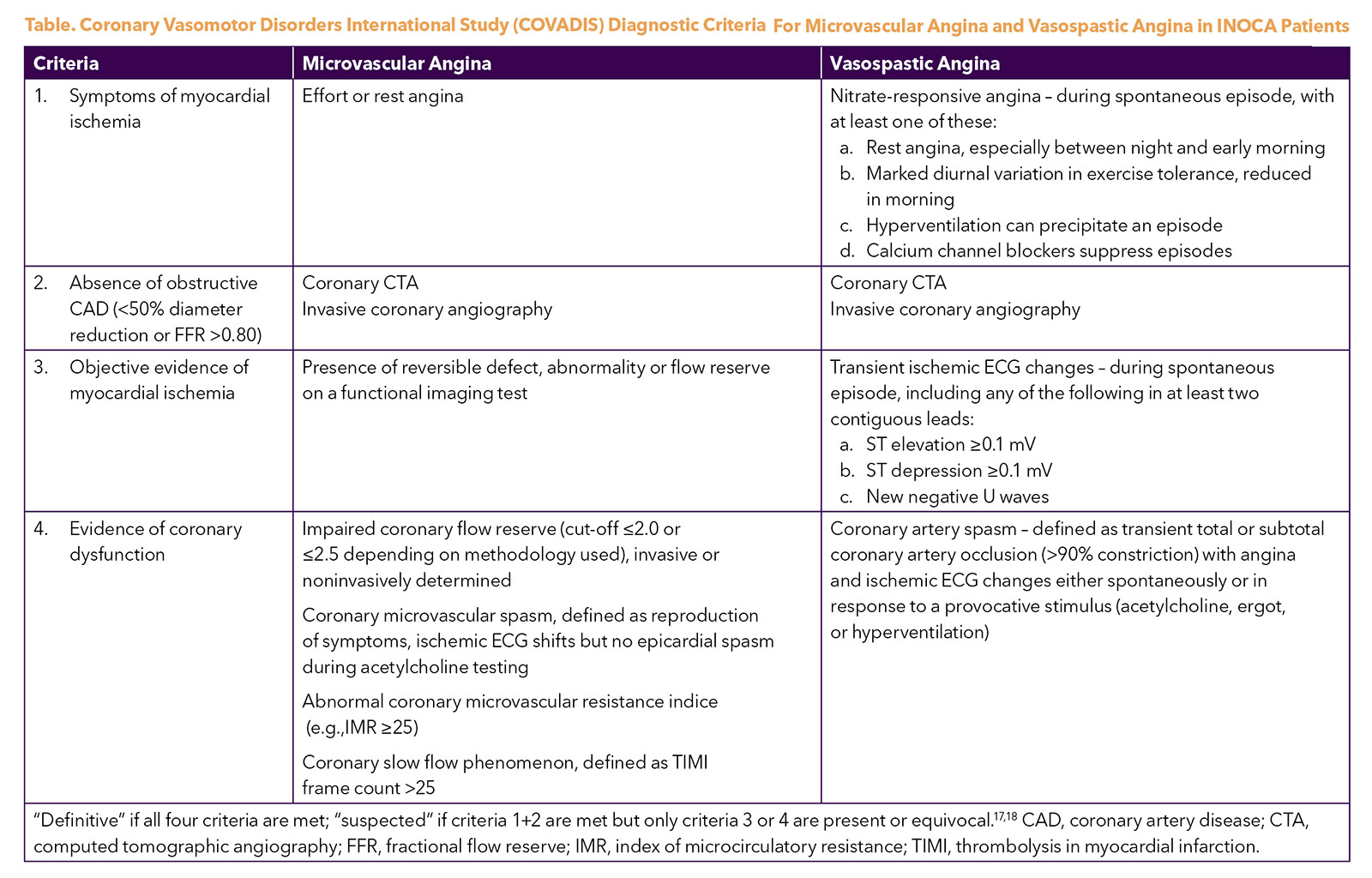
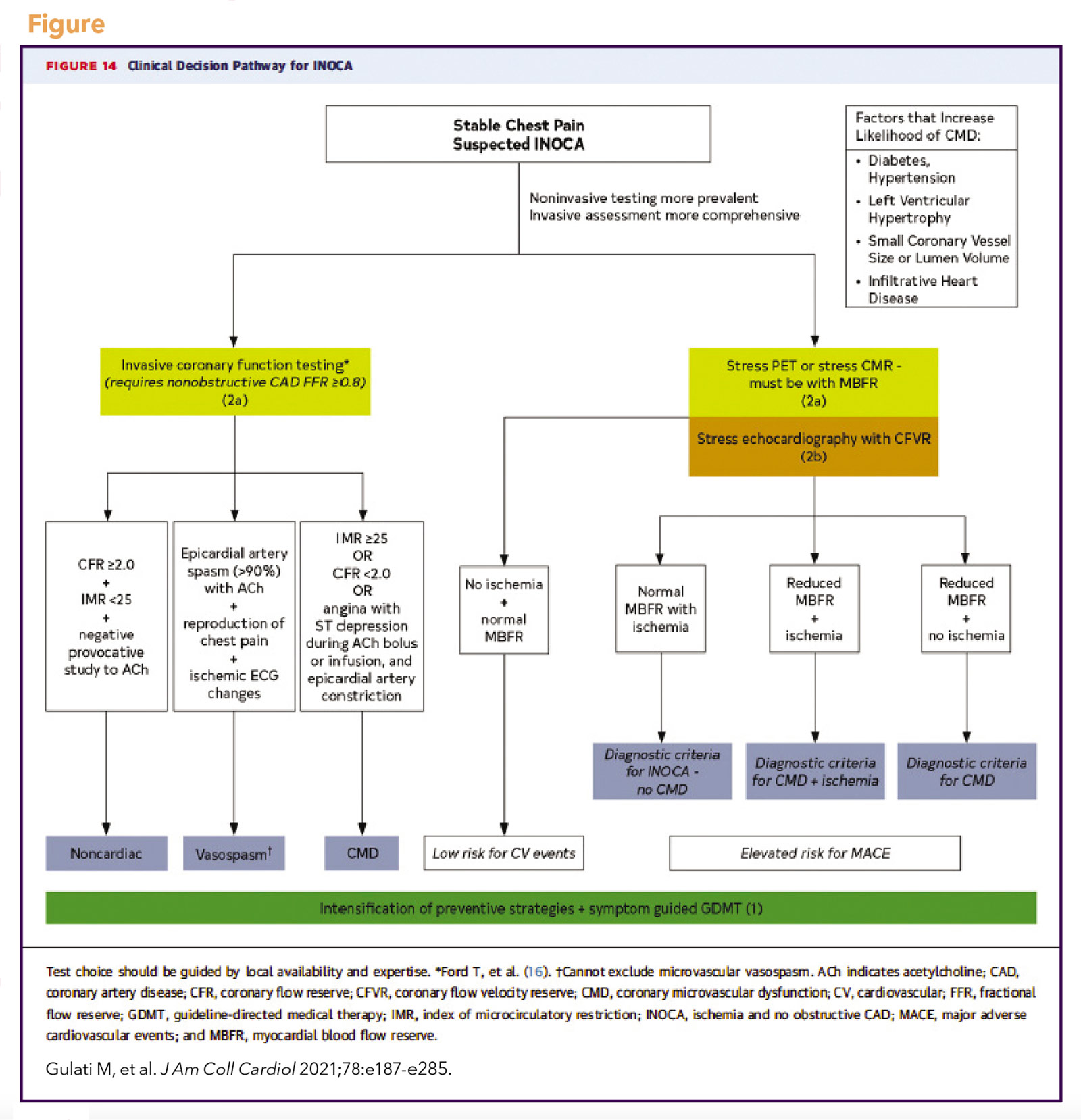
The Coronary Vasomotor Disorders International Study (COVADIS) study group was established in 2012 to develop international standards for the diagnostic criteria of microvascular and vasospastic angina (Table),17,18 now used in national and European guidelines.19,20 The 2021 ACC/AHA chest pain guideline adopted these definitions and proposed a diagnostic evaluation pathway for patients with stable chest pain and suspected INOCA (Figure), including noninvasive and invasive testing strategies.19 Test selection should be guided by local availability and expertise.
Noninvasive Testing of CMD
Clinical noninvasive diagnosis of CMD relies on identification of impaired coronary flow reserve (CFR) in the absence of flow-limiting CAD. Impaired CFR, calculated as the ratio of hyperemic to rest coronary blood flow (or myocardial blood flow), reflects flow abnormalities within the epicardial coronary arteries and microvasculature.21,22 Maximal hyperemia is induced with dipyridamole, adenosine or regadenoson. Caffeine and vasodilating medications are withheld for 24-48 hours prior to testing to avoid interference with pharmacologic stress. CMD can be measured by positron emission tomography (PET), myocardial flow reserve (MFR), cardiac magnetic resonance (CMR), myocardial perfusion reserve (MPR) and Doppler echocardiography coronary flow velocity reserve (CFVR).
Positron Emission Tomography
PET quantification of MFR has been highly validated in animals and humans, with standardized protocols and well-established reproducibility.23 Commonly used tracers are 82Rb-chloride and 13N-ammonia. Quality control of dynamic images and time-activity curves are critical, and list-mode acquisition is recommended for reconstruction of static, gated and dynamic datasets.23 Mean reference values for MFR in healthy young participants undergoing stress PET are 4.07 (for 82Rb PET) and 3.54 (for 13N-ammonia PET).23 Risk factors such as diabetes, hypertension, age, obesity and smoking may decrease MFR in the absence of obstructive CAD.24
Regardless of sex, PET MFR < portends increased risk of major adverse cardiac events (MACE) in patients without obstructive CAD9,25 and appears to be a stronger predictor of cardiovascular mortality than hyperemic myocardial blood flow (MBF) in patients with no obstructive CAD.26 Compared with patients with MFR >2, PET evidence supports that prognosis is worse regardless of whether MFR is low due to impaired hyperemic MBF or high resting MBF, the latter being more common among women than men.26
Cardiac Magnetic Resonance
CMR measures of MPR and its index (MPRI) have emerged as diagnostic and prognostic measures of CMD.27 Myocardial perfusion CMR should be acquired using standardized protocols under pharmacologic stress, with first-pass acquisition during rest and pharmacological stress.28
Quantitative evaluations require dual bolus, dual contrast sequences or other algorithms to correct for nonlinearity of signal intensity, correction for baseline signal differences, and efficient motion correction.
Standards for semiquantitative or fully quantitative methods have not been well defined due to availability of many different sequences and postprocessing parameters. Semiquantitative MPRI is calculated as the relative upslope of stress to rest, with relative upslope defined as the ratio between the maximum upslope of the first-pass myocardial perfusion time-intensity curve divided by the maximum upslope of the first-pass left ventricular cavity time-intensity curve.
Prior studies have demonstrated a semiquantitative MPRI <1.84 and quantitative MPR <2.19 to be thresholds with high sensitivity and specificity for invasively determined CMD.29,30 Both MPRI and MPR have been shown to provide risk stratification in patients with INOCA. MPRI is an independent predictor of MACE in patients with INOCA, with an MPRI ≤1.47 as the optimal prognostic threshold.31
Recently, a large quantitative CMR perfusion mapping study demonstrated MPR to be independently associated with mortality and MACE in patients with INOCA.32 CMR is a useful diagnostic tool in the evaluation of patients with myocardial infarction in the setting of no obstructive coronary arteries (MINOCA), particularly when acquired early after acute MINOCA.33-35
Doppler Echocardiography
Transthoracic Doppler echocardiography can assess CMD by measuring CFVR in the large coronary arteries. Coronary flow velocity measurements are typically performed in the mid-distal left anterior descending (LAD) due to its position near the chest wall. Using foreshortened two- and three-chamber views to locate the vessel, pulsed-wave Doppler is then used to measure mean or maximum diastolic coronary flow velocities averaged over three heart beats.36,37
CFVR is calculated as the ratio of stress to rest diastolic coronary flow velocity. Measurements including the surface anatomic position, degree of rotation of the transducer and LAD position should be carefully documented to ensure velocity measurements are in the same LAD segment at rest and hyperemia.
In a large study of women with suspected INOCA, CFVR predicted MACE, with an optimal cut-off value of CFVR <2.25 in the LAD.38
Invasive Coronary Function Testing of CMD, Vasospasm
Invasive coronary function testing is recommended for select patients with frequent or persistent stable angina and suspected INOCA as an alternative or adjunct to noninvasive testing.19,20 Similar with noninvasive testing, caffeine and vasodilating medications are withheld for 24-48 hours prior to avoid interference. As visualization of the microvasculature is beyond the resolution of angiography, its function is interrogated using pharmacologic agents, commonly adenosine and acetylcholine. While acetylcholine provocation is not commonly performed, it has been demonstrated to be safe with a low incidence of major complications.2,5,8,39,40
Coronary Flow Reserve
CFR can be invasively assessed with two techniques: 1) flow velocity using a Doppler wire, and 2) thermodilution using a pressure-temperature sensor guidewire. In the Doppler method, the Doppler wire is positioned in a straight segment of the coronary artery until a stable and high-quality Doppler flow signal is obtained. Doppler peak flow velocities are then averaged over three consecutive heartbeats to derive average peak velocity. Intravenous or intracoronary adenosine is administered to achieve hyperemia.
Doppler-derived CFVR is the ratio between hyperemic average peak velocity and resting average peak velocity and has high correlation with coronary volumetric flow reserve.41 CFVR in response to intravenous adenosine infusion may result in lower values compared to intracoronary bolus injections of adenosine.42
In the thermodilution method, an optimal and uniform saline bolus should be limited to 3-4 mL per injection.43 Intravenous adenosine is administered to achieve hyperemia.
Thermodilution CFR is measured as the ratio of mean transit time for saline to pass between the proximal and distal sensors at rest and at hyperemia, has been validated in animals and humans,44,45 and has modest correlation with Doppler CFVR.43
CFR measurements in healthy asymptomatic participants are usually >3.0, indicating that coronary circulation can triple coronary blood flow when required.21 CFR is generally lower in women compared with men, possibly due to sex differences in resting coronary flow.46,47
A CFR cutoff of <2.0 has been identified as significantly correlated with ischemia identified on SPECT imaging, with high sensitivity and specificity.48,49 Therefore, this is often considered the threshold for abnormal microcirculatory function,20,50 although recently a common threshold of <2.5 for both Doppler and thermodilution CFR has been proposed to optimize accuracy.43 Among women with suspected INOCA, CFR <2.32 was found to be the best discriminating threshold for MACE.10
Index of Microcirculatory Resistance
A pressure sensor/thermistor-tipped wire is used to calculate the index of microcirculatory resistance (IMR) at maximal hyperemia during intravenous adenosine infusion.51 IMR is defined as the hyperemic mean distal pressure multiplied by hyperemic mean transit time. An IMR >25 is considered abnormal.52 IMR is specific to the microvasculature and is not affected by changes in patient hemodynamics.
Doppler-derived hyperemic microvascular resistance (hMR) is defined as the ratio between hyperemic mean distal pressure and hyperemic average peak velocity. An hMR >2.4 is considered abnormal.53 The hMR correlates modestly with IMR and CFR.47,54 Patients may have discordant CFR and IMR due to differences in resting flow.55
Abnormal IMR in combination with low CFR has been associated with increased MACE in patients with INOCA, but an isolated elevated IMR did not portend worse outcomes.55
Epicardial and Microvascular Vasospasm
Epicardial and microvascular vasospasm may occur in the setting of smooth muscle hyperreactivity or endothelial dysfunction.56-58 Provocation of vasospasm is performed with an intracoronary injection of incremental doses of acetylcholine in the right and left coronary arteries, each administered over at least 20 seconds.53
Epicardial spasm is diagnosed when there is reduction in coronary diameter >90% following intracoronary acetylcholine with symptoms and ischemic electrocardiographic (ECG) changes. When angina and ischemic ECG changes occur without significant epicardial constriction (<90% reduction), then microvascular spasm is diagnosed.
Invasive provocative testing demonstrates that epicardial or microvascular spasm is highly prevalent in patients with suspected INOCA.59 Epicardial spasm is associated with an increased hazard of myocardial infarction and repeat angiography, while microvascular spasm is associated with recurrent angina.14 As nitrates are much less effective in preventing microvascular spasm than epicardial spasm, acetylcholine rechallenge after administration of nitroglycerin may be helpful in diagnosing coexistent microvascular spasm in patients with epicardial spasm.60
Coronary Endothelial Dysfunction
Although invasive coronary endothelial function testing is not mentioned in the ACC/AHA chest pain guideline,19 the diagnostic yield of coronary function testing is increased with additional endothelial function testing.61 While adenosine has partial endothelial effects,62 coronary endothelial dysfunction is typically defined as an attenuated increase or decrease in coronary blood flow (normal >50% increase) and coronary artery diameter (normal >5% dilation) in response to low dose acetylcholine.3,5
While endothelial dysfunction may affect both epicardial and microvascular coronary arteries, coronary blood flow may be preserved or even increased in the setting of coronary epicardial vasoconstriction in response to acetylcholine, suggesting preserved microvascular endothelial function in the setting of epicardial coronary endothelial dysfunction.63 Invasively determined coronary endothelial dysfunction portends increased MACE and mortality risk in patients with INOCA.13,64
Future Considerations
Accumulating evidence demonstrates the clinical benefit of identifying pathophysiologic mechanisms for diagnosis, prognosis and tailored medical therapy in INOCA patients. The recent ACC/AHA chest pain guideline recommends considering invasive and noninvasive tools for the diagnosis of INOCA endotypes and to enhance risk stratification (class 2a).19 Widespread adoption of these tools requires standardized protocols and reporting tools, which are increasingly available. Future directions include clinical trials using these diagnostic strategies to investigate therapeutic advances and to determine optimal therapy for each INOCA endotype and impact on long-term cardiovascular outcomes.


This article was authored by Phillip R. Tacon, MD, internal medicine resident at Cedars-Sinai Medical Center, and Janet Wei, MD, FACC, assistant professor at the Cedars-Sinai Smidt Heart Institute and Biomedical Imaging Research Institute, all in Los Angeles, CA.
Special Engagement Spotlight Series: Small Vessel Impact on Angina and Ischemia
The diagnosis of coronary artery disease (CAD) has focused on the presence of obstructive epicardial CAD. Nonetheless, it is estimated that at least two in every five male and female patients with angina referred for elective coronary angiography have nonobstructive epicardial coronary arteries, with rates relatively higher in women.
Myocardial ischemia does not require the presence of obstructive coronary arteries. This is recognized in the recent ACC/American Heart Association guideline for chest pain, expanding the definition of CAD to include both obstructive and nonobstructive CAD. The new guideline includes a diagnostic pathway for evaluation of chest pain for those with evidence of myocardial ischemia but no obstructive coronary arteries (INOCA), often due to small vessel angina due to ischemia. Small vessel angina and ischemia is a common but heterogenous condition associated with elevated risk for cardiovascular events. Diagnostic criteria for the two primary mechanisms, small vessel coronary microvascular dysfunction (CMD) and vasospasm, have been established. The combination of microvascular angina and epicardial vasospastic angina is associated with a more adverse prognosis. These mechanisms can also occur in the setting of epicardial obstructive CAD, further impairing prognosis.
Patients with small vessel ischemia and angina can pose both a diagnostic and therapeutic challenge to cardiologists. Many patients struggle for years to have an accurate diagnosis made, due to lack of physician awareness and expertise along with limited availability of diagnostic testing.
With consideration of small vessel ischemia and angina now included within ACC/AHA evidence-based guidelines, improving quality of care to reduce adverse cardiac event risk and enhance quality of life for patients is critical.
Over the course of the next four issues of Cardiology, you will hear from Janet Wei, MD, FACC, on making the diagnosis in the catheterization laboratory and noninvasively, using new guidelines and expert consensus approaches to establishing small vessel ischemia. In addition, Cindy L. Grines, MD, FACC, will outline the limitations of PCI for proximal obstruction and why small vessel testing can enhance approaches to ischemia and angina. Also learn more about distal vessel abnormality in ischemic patients from Timothy D. Henry, MD, FACC, including the rationale and techniques for combining revascularization with other physical therapies such as enhanced external counter-pulsation for ischemia and angina relief. Carl J. Pepine, MD, MACC, will round out the series with a review of treatment options including up-to-date medical therapies for small vessel ischemia and angina and discuss ongoing randomized clinical trials for this enlarging group of patients.
The entire series will be available at ACC.org/Cardiology as the series rolls out.

This Spotlight Series of articles was developed by invited Guest Editor, C. Noel Bairey Merz, MD, FACC, a leading expert in small vessel disease. She is professor of cardiology, director of the Barbra Streisand Women's Heart Center, director of the Linda Joy Pollin Women's Heart Health Program and director of the Preventive and Rehabilitative Cardiac Center, all at the Cedars-Sinai Smidt Heart Institute, in Los Angeles, CA.
References
- Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): Developing Evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075-92.
- Ford TJ, Stanley B, Good R ,et al. Stratified Medical therapy using invasive coronary function testing in angina: The CorMicA trial. J Am Coll Cardiol 2018;72:2841-55.
- Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445-53.
- Aziz A, Hansen HS, Sechtem U, et al. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol 2017;70:2349-58.
- Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646-53.
- Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054-60.
- Cassar A, Chareonthaitawee P, Rihal CS, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv 2009;2:237-44.
- Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014;129:1723-30.
- Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518-27.
- Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32.
- Tavella R, Cutri N, Tucker G, et al. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2016;2:117-24.
- Kenkre TS, Malhotra P, Johnson BD, et al. Ten-year mortality in the WISE study (Women's Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes 2017;10.
- AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol 2019;73:684-93.
- Seitz A, Gardezy J, Pirozzolo G, et al. Long-term follow-up in patients with stable angina and unobstructed coronary arteries undergoing intracoronary acetylcholine testing. JACC Cardiovasc Interv 2020;13:1865-76.
- Takagi Y, Yasuda S, Tsunoda R, et al. Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol 2011;4:295-302.
- Ford TJ, Stanley B, Sidik N, et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv 2020;13:33-45.
- Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565-68.
- Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16-20.
- Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;78:e187-e285.
- Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504-20.
- Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging 2009;2:1009-23.
- Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639-53.
- Murthy VL, Bateman TM, Beanlands RS, et al. Clinical quantification of myocardial blood flow using PET: Joint position paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Cardiol 2018;25:269-97.
- Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2625-41.
- Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215-24.
- Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325-36.
- Patel AR, Salerno M, Kwong RY, et al. Stress cardiac magnetic resonance myocardial perfusion imaging: JACC Review Topic of the Week. J Am Coll Cardiol 2021;78:1655-68.
- Puntmann VO, Valbuena S, Hinojar R, et al. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: part I - analytical validation and clinical qualification. J Cardiovas Magnetic Resonance 2018;20:67.
- Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;8.
- Rahman H, Scannell CM, Demir OM, et al. High-resolution cardiac magnetic resonance imaging techniques for the identification of coronary microvascular dysfunction. JACC Cardiovasc Imaging 2021;14:978-86.
- Zhou W, Lee JCY, Leung ST, et al. Long-term prognosis of patients with coronary microvascular disease using stress perfusion cardiac magnetic resonance. JACC Cardiovasc Imaging 2021;14:602-11.
- Knott KD, Seraphim A, Augusto JB, et al. The prognostic significance of quantitative myocardial perfusion: an artificial intelligence-based approach using perfusion mapping. Circulation 2020;141:1282-91.
- Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021;143:624-40.
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: A scientific statement from the American Heart Association. Circulation 2019;139:e891-e908.
- Sorensson P, Ekenback C, Lundin M, et al. Early Comprehensive cardiovascular magnetic resonance imaging in patients with myocardial infarction with nonobstructive coronary arteries. JACC Cardiovasc Imaging 2021;14:1774-83.
- Schroder J, Prescott E. Doppler echocardiography assessment of coronary microvascular function in patients with angina and no obstructive coronary artery disease. Front Cardiovasc Med 2021;8:723542.
- Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol 2013;61:723-7.
- Schroder J, Michelsen MM, Mygind ND ,et al. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive coronary artery disease: results from the iPOWER study. Eur Heart J 2021;42:228-39.
- Reriani M, Sara JD, Flammer AJ, et al. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis 2016;27:213-20.
- Takahashi T, Samuels BA, Li W, et al. Safety of provocative testing with intracoronary acetylcholine and implications for standard protocols. J Am Coll Cardiol 2022;79:2367-78.
- Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 1999;33:1469-75.
- AlBadri A, Sharif B, Wei J, et al. Intracoronary bolus injection versus intravenous infusion of adenosine for assessment of coronary flow velocity reserve in women with signs and symptoms of myocardial ischemia and no obstructive coronary artery disease. JACC Cardiovasc Interv 2018;11:2125-7.
- Demir OM, Boerhout CKM, de Waard GA, et al. Comparison of doppler flow velocity and thermodilution derived indexes of coronary physiology. JACC Cardiovasc Interv 2022;15:1060-70.
- De Bruyne B, Pijls NH, Smith L,et al. Coronary thermodilution to assess flow reserve: experimental validation. Circulation 2001;104:2003-6.
- Pijls NH, De Bruyne B, Smith L, et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation 2002;105:2482-6.
- Kobayashi Y, Fearon WF, Honda Y, et al. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1433-41.
- Kumar S, Mehta PK, Eshtehardi P, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter Cardiovasc Interv 2021;98:827-35.
- Miller DD, Donohue TJ, Younis LT, et al. Correlation of pharmacological 99mTc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation 1994;89:2150-60.
- Meuwissen M, Siebes M, Chamuleau SA, et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation 2002;106:441-6.
- Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006;114:1321-41.
- Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129-32.
- Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: The index of microcirculatory resistance. Circ Cardiovasc Interv 2017;10.
- Ford TJ, Ong P, Sechtem U, et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: Why, how, and when. JACC Cardiovasc Interv 2020;13:1847-64.
- Williams RP, de Waard GA, De Silva K, et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol 2018;121:1-8.
- Lee JM, Jung JH, Hwang D, et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 2016;67:1158-69.
- Ohba K, Sugiyama S, Sumida H, et al. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc 2012;1:e002485.
- Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation 2011;124:1774-82.
- Hubert A, Seitz A, Pereyra VM, et al. Coronary artery spasm: The interplay between endothelial dysfunction and vascular smooth muscle cell hyperreactivity. Eur J Cardiol 2020;15:e12.
- Mileva N, Nagumo S, Mizukami T, et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: Systematic review and meta-analysis. J Am Heart Assoc 2022;11:e023207.
- Seitz A, Feenstra R, Konst RE, et al. Acetylcholine rechallenge: A first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovasc Interv 2022;15:65-75.
- Feenstra RGT, Boerhout CKM, Woudstra J, et al. Presence of Coronary endothelial dysfunction, coronary vasospasm, and adenosine-mediated vasodilatory disorders in patients with ischemia and nonobstructive coronary arteries. Circ Cardiovasc Interv 2022:15:e012017.
- Smits P, Williams SB, Lipson DE, et al. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation 1995;92:2135-41.
- Hasdai D, Gibbons RJ, Holmes DR Jr., Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997;96:3390-5.
- Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948-54.
Clinical Topics: Acute Coronary Syndromes, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Stable Ischemic Heart Disease, Vascular Medicine, Interventions and ACS, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Chronic Angina
Keywords: ACC Publications, Cardiology Magazine, Acute Coronary Syndrome, Care Team, Patient Care Team, Angiography, Diagnostic Imaging, Aneurysm, Exercise Test, Angina, Stable, Cardiology Magazine Spotlight Series
< Back to Listings