Peripheral Matters | Renal Denervation For Hypertension: Current Evidence and Future Directions
Hypertension is the leading cause of global death and disability, and the persistence of inadequate treatment and control of elevated blood pressure (BP) portends an ominous public health impact. In the U.S. alone, for example, more than half of individuals with hypertension are estimated to have BP exceeding professional society- and guideline-recommended treatment goals.1
This stark reality – combined with results from large, randomized trials demonstrating the benefits of more intensive BP lowering2 – motivates the development of device-based therapies such as renal denervation (RDN) for hypertension as a complement to pharmaceutical therapies and lifestyle interventions.
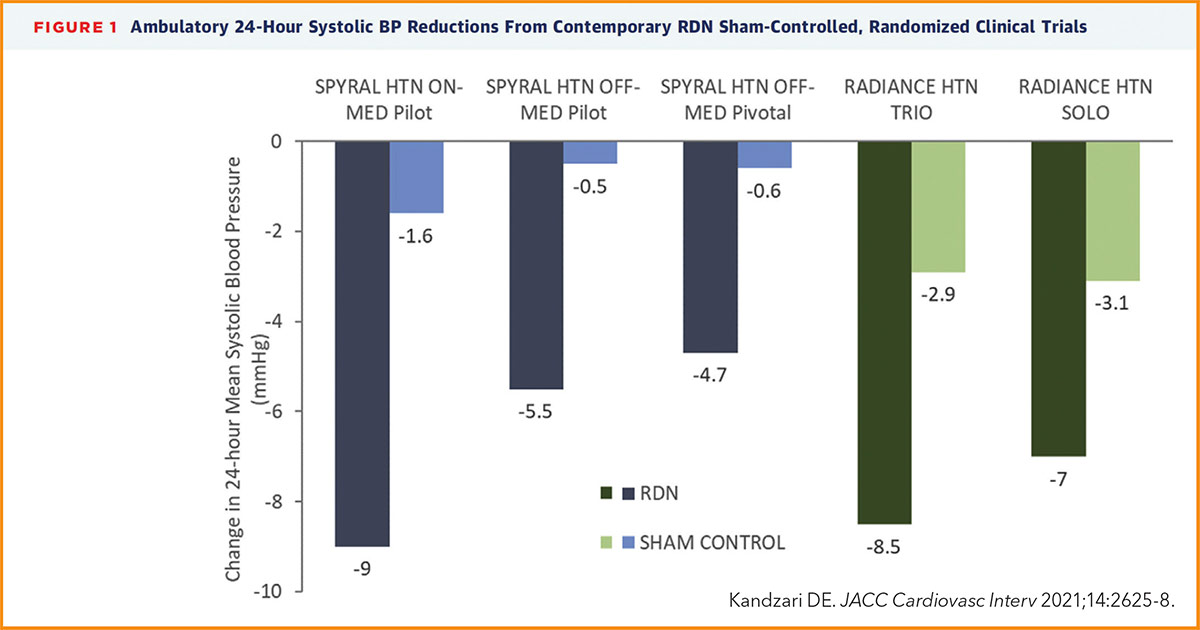
Early clinical trial experience related to procedural technique, medication variability and both patient and endpoint selection have informed the revision of trial design and conduct leading to a new generation of randomized, sham-controlled trials involving RDN.3 Overall, these studies have demonstrated significant BP reductions following RDN using two different treatment methods, both in the presence and absence of antihypertensive drug therapy (Figure 1).4-8
Following demonstration of significant BP reductions with RDN in the randomized, sham-controlled SPYRAL HTN OFF-MED and ON-MED pilot trials,4,5 the SPYRAL HTN OFF-MED Pivotal trial of similar trial design was statistically powered to evaluate the efficacy of catheter-based radiofrequency energy RDN in the absence of antihypertensive therapy,6 an approach similar with the evaluation of novel antihypertensive medications.
In the SPYRAL HTN OFF-MED Pivotal trial, hypertensive patients with an office systolic BP ≥150 mm Hg and <180 mm Hg (average systolic and diastolic BP 163 mm Hg and 102 mm Hg, respectively) were evenly randomized to RDN or sham control to evaluate the primary and secondary effectiveness endpoints of differences in 24-hour and office systolic BP at three months of follow-up.
Among a study population of 331 randomized patients, the primary and secondary effectiveness endpoints were met, favoring RDN, with a between-group treatment difference of –3.9 mm Hg in 24-hour systolic BP and –6.5 mm Hg in office systolic BP, and a Bayesian posterior probability of superiority >0.99 for both endpoints.
More specifically, RDN was associated with absolute reductions of 4.7 mm Hg and 9.2 mm Hg for 24-hour and office systolic BP, respectively. Treatment differences in 24-hour and office diastolic pressure at three months were also significantly lower with RDN. Additionally, treatment differences were consistent among prespecified subgroups with no significant interactions observed. Moreover, there were no major device- or procedural-safety events through three months.
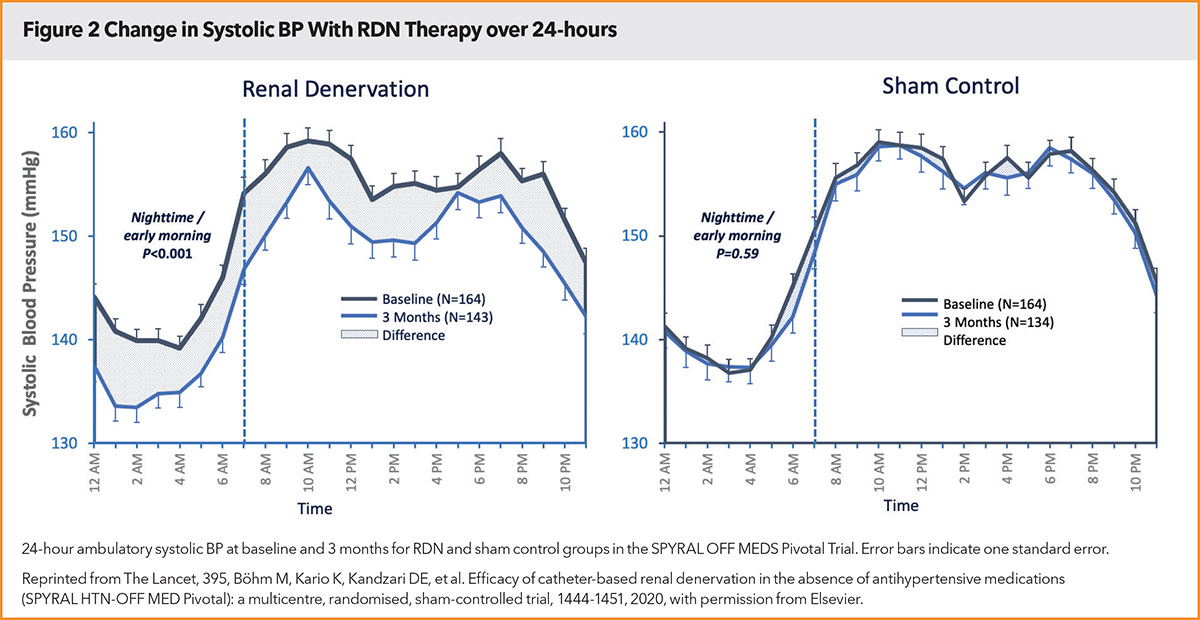
As the largest randomized evaluation of RDN in the absence of antihypertensive medications, the SPYRAL HTN OFF-MED Pivotal trial confirms the effectiveness of this method to reduce BP and provides further reassurance related to safety specific to this technology and technique. Consistent with prior studies, the persistent reduction ('always on' effect, Figure 2) in BP achieved with RDN over a 24-hour period distinguishes this therapy from limitations associated with medications, including varied pharmacokinetic profiles and dosing regimens as well as drug adherence. Further, the consistent reduction in BP at all time points may have special relevance for individuals whose BP phenotype conveys high cardiovascular risk, for example, those with nocturnal or early morning hypertension.
More Studies in Treated, Uncontrolled HTN
Aside from the SPYRAL HTN OFF-MED Pivotal trial, additional study is both completed and ongoing in patients with uncontrolled hypertension despite prescribed medical therapy, a setting more representative of clinical practice for which integrating drug and procedural strategy may be anticipated.
In the sham-controlled, randomized SPYRAL HTN ON-MED pilot study (n=80), RDN was associated with significant reductions in both office and ambulatory systolic and diastolic BP at six months among patients treated with one to three antihypertensive drugs of different classes.5
More recently, the RADIANCE TRIO study randomized 136 patients with treatment-resistant hypertension despite three antihypertensive medications to RDN using IVUS or sham control; at two months, both ambulatory and office systolic BP measures were significantly reduced with RDN (24-hour ambulatory BP difference, –4.2 mm Hg; p=0.016).8
At present, the SPYRAL ON-MED extension trial is ongoing to examine the effectiveness of RDN in a larger population with uncontrolled hypertension despite prescription of antihypertensive medications (NCT02439775). In parallel, the TARGET BP 1 trial is enrolling patients with a similar design, yet with perivascular alcohol delivery to achieve renal efferent and afferent denervation (NCT02910414).
Integrating RDN in Clinical Practice
Given the proven therapeutic potential of RDN, focus has more recently shifted beyond proof of concept to issues relevant to clinical application. Although current studies have not been designed to compare long-term clinical outcomes, one issue of interest relates to the clinical relevance of BP reductions observed with RDN. In studies of pharmaceutical therapies, a 10 mm Hg decline in office systolic BP – similar with that observed in contemporary RDN trials – is associated with approximately 25% relative reductions in stroke and cardiac events, in addition to nearly a 15% reduction in mortality.9
In a recent meta-analysis of 51 randomized trials evaluating antihypertensive medications, an absolute systolic BP reduction of 5 mm Hg was achieved with a single antihypertensive drug,10 a difference that translates to an approximate 5% relative reduction in cardiovascular death and 10-15% reductions in cardiovascular events and stroke. Recent meta-analyses of existing randomized trials demonstrated that RDN is associated with a BP reduction at least similar with that of a single-pill dual-agent combination,11 and may translate to estimated relative reductions of 10% in lifetime risk of cardiovascular events and 7.5% in all-cause mortality.12
A focus for further studies is to address the durability of BP lowering and a reduction in antihypertensive medications, as well as late-term safety and the impact on end organ function.
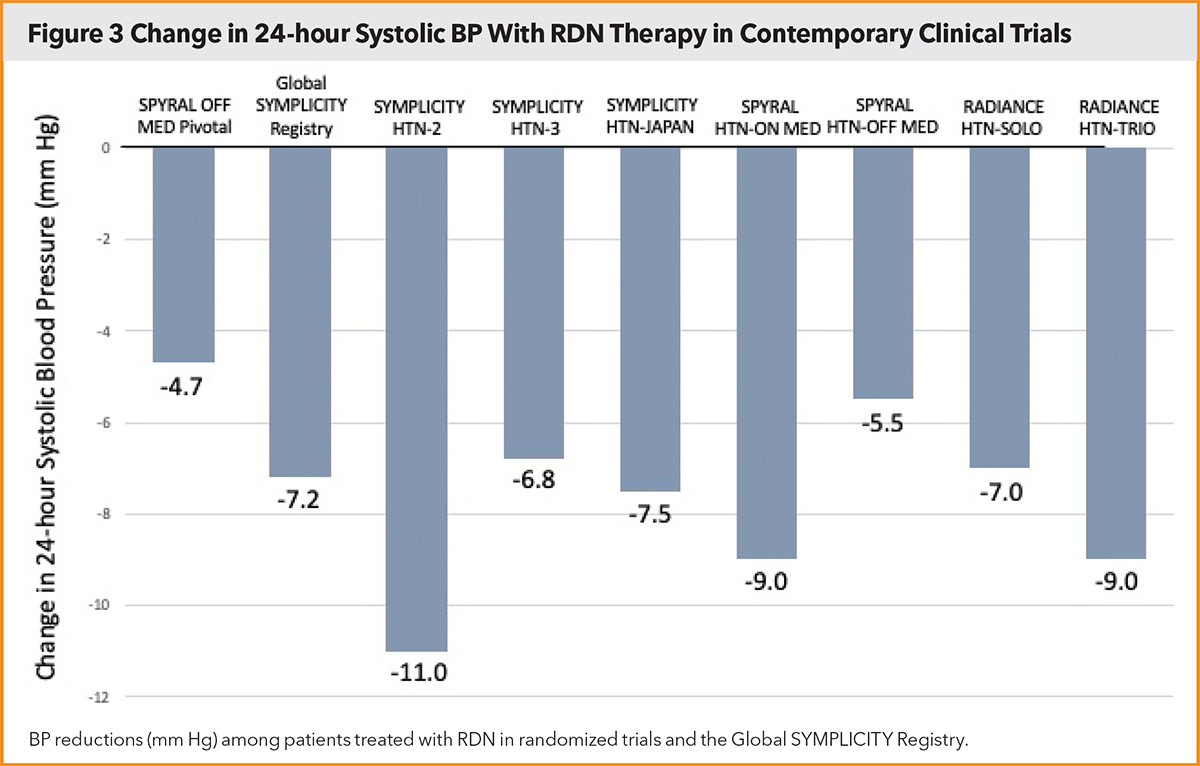
Progressive lowering of BP over longer timelines has been observed following RDN.5 The largest evidence currently related to durability is derived from the Global SYMPLICITY Registry among a broad, unselected patient population with uncontrolled hypertension.13 Through three years of follow-up, RDN was associated with sustained and significant reductions in both office and ambulatory BP, independent of an escalation in medication burden. In addition to clinically meaningful reductions in BP following RDN (Figure 3), additional studies have demonstrated achievement of targeted BP measures with fewer medications and/or reduced dosages compared with a control group.14,15
Regarding safety following RDN, randomized trials have not demonstrated an increased risk of vascular complications, decline in renal function, or later identification of renal artery stenosis.16
Patient-Centered Focus
Considering the integration of RDN into standard treatments for hypertension, identifying predictors of treatment effect and surveys of patient-reported outcomes and patient preferences are also evolving areas of investigation. Effectiveness in patients with clinical characteristics excluded from randomized trials having more restrictive enrollment criteria is also a focus of attention.
In the Global SYMPLICITY Registry, a similar magnitude of BP reduction has been observed following RDN among patients with varying cardiovascular risk and comorbidities.17 Such issues of interest also will be addressed in real-world registries inclusive of patients with hypertension representative of clinical practice, and with dedicated long-term follow-up (AFFIRM and Global Paradise [NCT05027685] Registries). Aside from confirmatory studies of safety and effectiveness, these additional studies will further inform patient selection, expand experience with RDN in 'real world' clinical practice, and provide guidance for how RDN may be incorporated into treatment pathways.18,19
Altogether, results of contemporary RDN studies not only reaffirm the biologic proof of principle for this novel treatment, but also offer insight to its complementary benefit in uncontrolled hypertension despite prescribed medications.
Across studies of varied design and even with different methods, consistent and clinically meaningful reductions in BP have been achieved with RDN. With this momentum, additional studies are intended to position RDN as part of standard therapy for the world's leading cause of death and disability.
Identifying predictors of treatment effect is an evolving area of focus to inform patient selection and expectations. Equally important, studies are underway to demonstrate durability of BP lowering amidst unpredictable patient and prescriber behavior and coexisting health conditions that may influence BP.
Finally, with awareness of a high prevalence of medication nonadherence in hypertension treatment, patient preference and tolerance of the risk/benefit balance with both medications and device-based therapy represent an important focus of forthcoming study.

This article was authored by David E. Kandzari, MD, FACC, chief of the Piedmont Heart Institute in Atlanta, GA, where he is also director of interventional cardiology. Reach him on Twitter @Kandzari. He reports institutional research/grant support and consulting honoraria from Medtronic and Ablative Solutions.
References
- Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA 2020;324:1190-1200.
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103-16.
- Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension 2020;76:1410-17.
- Townsend RR, Mahfoud F, Kandzari DE, et al; SPYRAL HTN OFF MED Trial Investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160-70.
- Kandzari DE, Böhm M, Mahfoud F, et al; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018;391:2346-55.
- Böhm M, Kario K, Kandzari DE, et al; SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020;395:1444-51.
- Azizi M, Schmieder RE, Mahfoud F, et al; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335-45.
- Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021;397:2476-86.
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67.
- Blood Pressure Lowering Treatment Trialists' Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021;39:1625-36.
- Salam A, Kanukula R, Atkins E, et al. Efficacy and safety of dual combination therapy of blood pressure-lowering drugs as initial treatment for hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens 2019;37:1768-74.
- Ahmad Y, Kane C, Arnold AD, et al. Randomized blinded placebo-controlled trials of renal sympathetic denervation for hypertension: a meta-analysis. Cardiovasc Revas Med 2022;34:112-8.
- Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J 2019;40:3474-82.
- Azizi M, Schmieder RE, Mahfoud F, et al. Six-month results of treatment-blinded medication titration for hypertension control after randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation 2019;139:2542-53.
- Kandzari DE, Hickey GL, Pocock SJ, et al. Prioritised endpoints for device-based hypertension trials: the win ratio methodology. EuroIntervention 2021;16:e1496–e1502.
- Townsend RR, Walton A, Hettrick DA, et al. Review and meta-analysis of renal artery damage following percutaneous renal denervation with radiofrequency renal artery ablation. EuroIntervention 2020;16:89–96.
- Mahfoud F, Mancia G, Schmieder R, et al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol 2020;75:2879–2888.
- Kandzari DE, Townsend RR, Bakris G, et al. Renal denervation in hypertension patients: Proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv 2021 Aug 3. doi: 10.1002/ccd.29884.
- Schmieder RE, Mahfoud, F, Mancia G, et al., on behalf of members of the ESH Working Group on Device-Based Treatment of Hypertension. European Society of Hypertension position paper on renal denervation 2021. J Hypertension 2021;39:1733-1741.
- Kandzari DE. Mirror, mirror on the wall: Who is the fairest meta-analysis of all? JACC Cardiovasc Interv 2021;14:2625-8.
Clinical Topics: Cardiovascular Care Team, Prevention, Hypertension
Keywords: ACC Publications, Cardiology Magazine, Antihypertensive Agents, Blood Pressure, Bayes Theorem, Goals, Hypertension, Pharmaceutical Preparations, Life Style, Technology, Catheters
< Back to Listings