Focus on Heart Failure | Thinking Outside the Ice Box: Preservation Techniques, New Technologies in Transplantation
Transplantation is lifesaving for many of our patients. Yet an ongoing challenge is how to expand this opportunity to all patients who need a heart in our current setting of limited resources. While 2023 was the first year that more than 4,000 transplants were performed (at a total of 4,545) in the U.S., clearly organ availability remains limited compared to the number of patients who could benefit from the therapy.
A recent patient, Ms. A, helps to illustrate some of the challenges. A 48-year-old woman with nonischemic cardiomyopathy, she developed advanced heart failure (HF) despite optimal medical therapy. She was listed for heart transplantation. Her blood type is O. Because of previous pregnancies, she is sensitized due to the presence of pre-formed antibodies to human leukocyte antigens (HLA). Despite being supported with an intra-aortic balloon pump as Status 2, a long wait time due to her sensitization and blood type was expected.
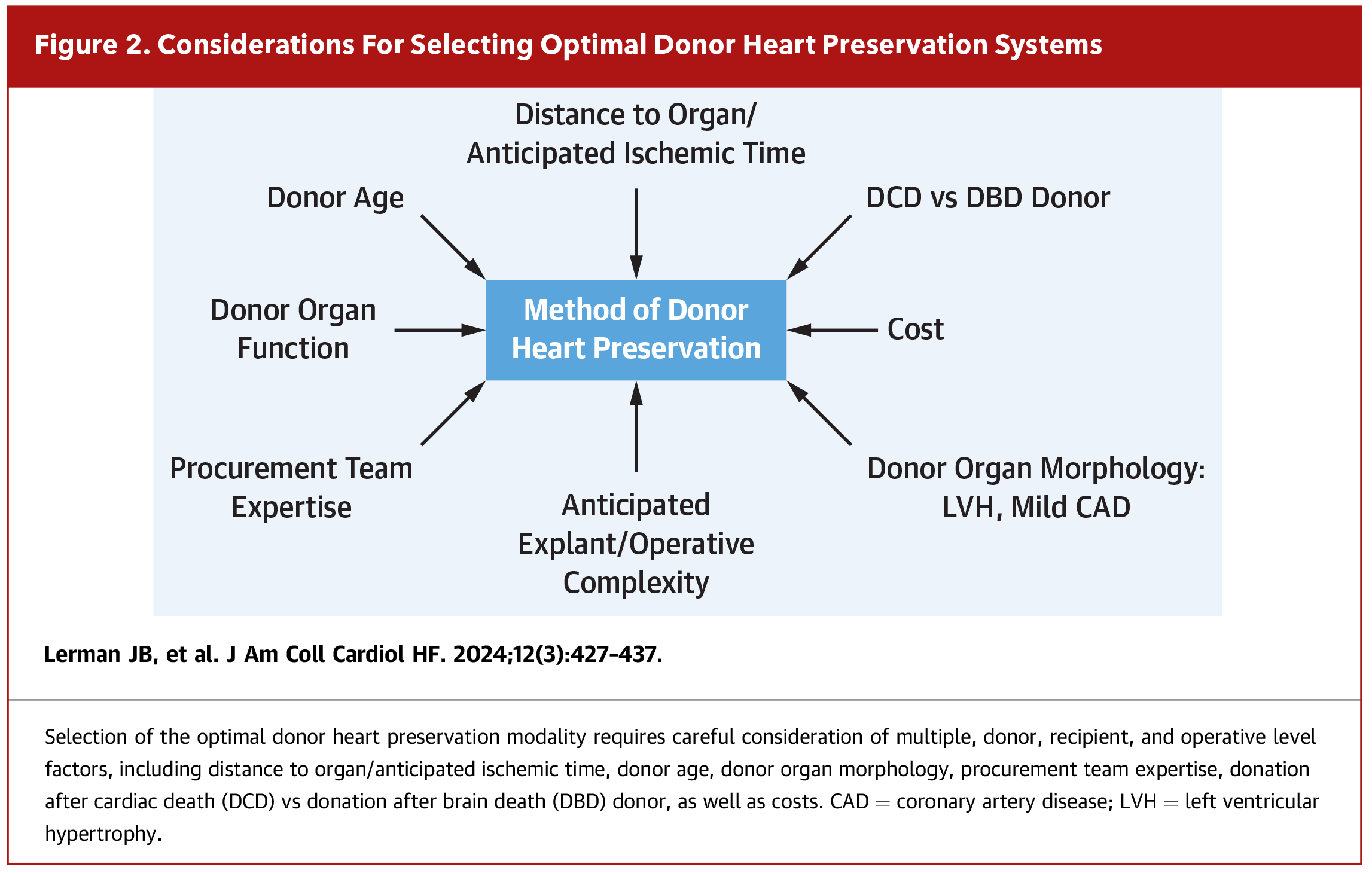
Unfortunately, cases like Ms. A's are not uncommon. Although the number of heart transplants is increasing, organ availability remains limited compared to the number of patients who could benefit from the therapy. Following the implementation of the new allocation system in 2018, geographical sharing has led to increased ischemic times and greater distance traveled for organs.1 Fortunately, various innovations in the field of preservation have helped to expand opportunities for transplant. Figure 1 highlights some of these advances and Figure 2 highlights considerations for selecting the optimal technology.

Paragonix SherpaPak CTS
The traditionally standard approach for organ transplant has been preservation using a three-bag technique and storage in an ice cooler.2 However, temperature monitoring can be unpredictable leading to uneven cooling and potential tissue injury. More recently, there has been increasing use of the Paragonix SherpaPak Cardiac Transport System (CTS) as an alternative to ice storage. It is approved by the U.S. Food and Drug Administration (FDA) for transport for organ transplantation. The SherpaPak involves a single-use box with temperature that is closely regulated between 4 and 8 degrees Celsius.3
The ongoing GUARDIAN (Global Utilization and Registry Database for Improved Heart Preservation) Registry, currently with more than 1,900 patients, will provide important insights and answers to clinical questions. Data from GUARDIAN presented at the International Society for Heart and Lung Transplantation meeting in 2024 demonstrated that the use of the SherpaPak CTS has been associated with significant improvement in survival at two years compared to ice after propensity matching (94.3% vs. 89.5%, p=0.04).4 Approximately half of all donation-after-breath-death (DBD) donor hearts in 2023 were transported using the SherpaPak. Additional findings include a significant reduction in severe primary graft dysfunction (PGD) by 51% (p=0.009).
SheraPak CTS has also allowed for longer procurement distances and a reduction in PGD in cases of prolonged ischemic time (considered more than four hours). In one case, the SherpaPak was used to transport a donor heart nearly 3,000 miles with an ischemic time of over seven hours.5 In this way, this technology may help to facilitate transplantation among women like Ms. A who may need a broader geographic range to find a suitable donor.
Transmedics OCS
Another alternative to cold storage that has emerged is the Transmedics OCS ex vivo heart perfusion technology, the only technology of its kind that is FDA approved. This allows for "beating heart" transportation of donor hearts, also nicknamed "Heart in a Box."
Once the heart has been procured, it is re-perfused with oxygenated, warmed, nutrient-enriched blood from the donor.1,6 The technology allows for continuous monitoring of the organ during transport, including hemodynamic parameters such as aortic pressure, coronary flow rate, oxygen saturation, venous and artery lactate levels, and direct visualization of graft contractility.
Transmedics OCS was compared to traditional cold storage in the Proceed II (Randomized Study of OCS for Preservation of Donor Hearts for EventuaL Transplantation study.7 Survival at 30 days was similar between groups. Subsequently the EXPAND (International Trial to Evaluate the Safety and Effectiveness of the Portable OCS Heart for Preserving and Assessing Expanded Criteria Donor Hearts for Transplantation) examined the use of OCS in expanded criteria donors (ECD), donors who are thought to be higher risk.8 These may include donors of older age, those with longer down time, thicker walls and other characteristics for which they may be declined transplantation. During the EXPAND trial, 86% of ECD hearts were able to be successfully transplanted using the OCS system.8 On average, these hearts had been declined 60 times prior to transplantation. Survival was 89% at one year and 85% at two years, similar to national registry report data for standard heart transplant recipients.8
The rise of OCS has likewise facilitated an increase in donation-after-circulatory-death (DCD). DCD donation refers to donors who do not meet criteria for legal brain death (DBD donors) but are neurologically devastated with no meaningful chance for recovery.2 In the setting of controlled withdrawal of life support, the organ is attempted to be procured after cardiac arrest. As previously mentioned, the OCS system allows for close monitoring of the organ as it is re-perfused to determine if it will be suitable for transplant.
In the DCD Heart Trial, a multicenter randomized controlled trial comparing patients who received DCD donors preserved with OCS with patients who received DBD hearts preserved with cold storage, DCD OS was noninferior to DBD cold storage with respect to survival at six months and one year.9
XVIVO Heart Preservation Device
Outside the US., ex vivo nonischemic heart transplantation (NIHP) has been shown to be safe and feasible using the XVIVO heart preservation device.10 In one phase 2 study of 42 patients who underwent transplant, six were assigned to NIHP and compared to those who underwent static cold preservation. There were no deaths or cardiac-related adverse events in the NIHP group.10 The randomized controlled multicenter trial of the XVIVO heart preservation device is ongoing and we look forward to the results.
Turning the Tide
With these exciting developments, it's a great time for our field of HF and transplant cardiology. Within the HF and transplant community, we hope these new techniques can help facilitate acceptance of donors who would previously have been determined to be unsuitable or too far away. Ms. A was fortunate enough to be at a DCD center with OCS technology. Although ischemic time was six hours, she did well without any evidence of PGD.

This article was authored by Ersilia M. DeFilippis, MD, FACC, an Advanced Heart Failure and Transplant Cardiology specialist and assistant professor of medicine, Division of Cardiology, Center for Advanced Cardiac Care, at Columbia University Irving Medical Center-New York Presbyterian Hospital.
References
- Lerman JB, Agarwal R, Patel CB, et al. Donor Heart Recovery and Preservation Modalities in 2024. JACC Heart Fail 2024;12:427-437.
- DeFilippis EM, Khush KK, Farr MA, et al. Evolving Characteristics of Heart Transplantation Donors and Recipients: JACC Focus Seminar. J Am Coll Cardiol 2022;79:1108-1123.
- Radakovic D, Karimli S, Penov K, et al. First Clinical Experience with the Novel Cold Storage SherpaPakTM System for Donor Heart Transportation. J Thorac Dis 2020;12:7227-7235.
- Silvestry S, Meyer D, Pham S, et al. Improved 2-Year Heart Transplant Survival with Moderate Hypothermic Donor Heart Preservation in the Guardian Heart Registry. J Heart Lung Transplant 2024;43:S67–S68.
- Li SS, Michel E, Osho AA, et al. Transcontinental Heart Transplant Using Sherpapak Cold Static Storage System. JHLT Open 2024;4:100062.
- García Sáez D, Zych B, Sabashnikov A, et al. Evaluation of the Organ Care System in Heart Transplantation with an adverse Donor/Recipient Profile. Annals Thoracic Surg 2014;98:2099-2106.
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo Perfusion of Donor Hearts for Human Heart Transplantation (PROCEED II): A Prospective, Open-Label, Multicentre, Randomised Non-Inferiority Trial. The Lancet 2015;385:2577-2584.
- Schroder JN, Patel CB, DeVore AD, et al. Increasing Utilization of Extended Criteria Donor Hearts for Transplantation. JACC: Heart Fail 2024;12:438-447.
- Schroder JN, Patel CB, DeVore AD, et al. Transplantation Outcomes with donor Hearts After Circulatory Death. N Engl J Med 2023;388:2121-2131.
- Nilsson J, Jernryd V, Qin G, et al. A Nonrandomized Open-Label Phase 2 Trial Of Nonischemic Heart Preservation for Human Heart Transplantation. Nat Commun 2020;11:2976.
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Cardiac Surgery and Heart Failure, Heart Transplant, Interventions and Vascular Medicine
Keywords: Cardiology Magazine, ACC Publications, Heart Transplantation, Tissue Donors, Primary Graft Dysfunction, Cryopreservation, Organ Transplantation