Peripheral Matters | Renal Denervation for Hypertension: Evolution of Evidence and Clinical Standards
Widespread enthusiasm for renal denervation (RDN) poised as a breakthrough therapy for uncontrolled hypertension was followed by abrupt disillusionment after the lack of demonstrable efficacy compared with antihypertensive therapy alone in the SYMPLICITY HTN 3 trial.1
From that experience, however, revision of trial design and conduct related to confounding variables of procedural technique, medication variability, and selection of both patients and endpoints has substantially informed contemporary study.2
With renewed interest and cautious perseverance, recent early phase, randomized, sham-controlled trials have now demonstrated significant blood pressure reductions following RDN in hypertensive patients, both in the presence and absence of antihypertensive drug therapy.3-5
As a pivotal study, the randomized, sham-controlled SPYRAL HTN OFF-MED trial was statistically powered to evaluate the efficacy of catheter-based RDN in the absence of antihypertensive therapy,6 an approach similar with the evaluation of antihypertensive medications.
Patients with hypertension with an office systolic blood pressure ≥150 mm Hg and <180 mm Hg (average systolic and diastolic blood pressures approximately 163 mm Hg and 102 mm Hg, respectively) were evenly randomized to RDN or sham control to evaluate the primary and secondary effectiveness endpoints of differences in 24-hour and office systolic blood pressure at the three-month follow-up.
The analysis in SPYRAL HTN OFF-MED utilized a Bayesian study design to combine data from the present trial (n=251) with an informative previous randomized pilot trial (n=80) and constitute the overall primary analysis population of 331 randomized patients.
The primary and secondary effectiveness endpoints were met, with a treatment difference of –3.9 mm Hg (Bayesian 95% credible interval, –6.2 to –1.6) in 24-hour systolic blood pressure and –6.5 mm Hg (–9.6 to –3.5) in office systolic blood pressure between groups, and a posterior probability of superiority >0.99 for both endpoints.7
More specifically, RDN was associated with absolute reductions of 4.7 mm Hg and 9.2 mm Hg for 24-hour and office systolic blood pressure, respectively. Treatment differences in 24-hour and office diastolic pressure at three months were also significantly lower with RDN.
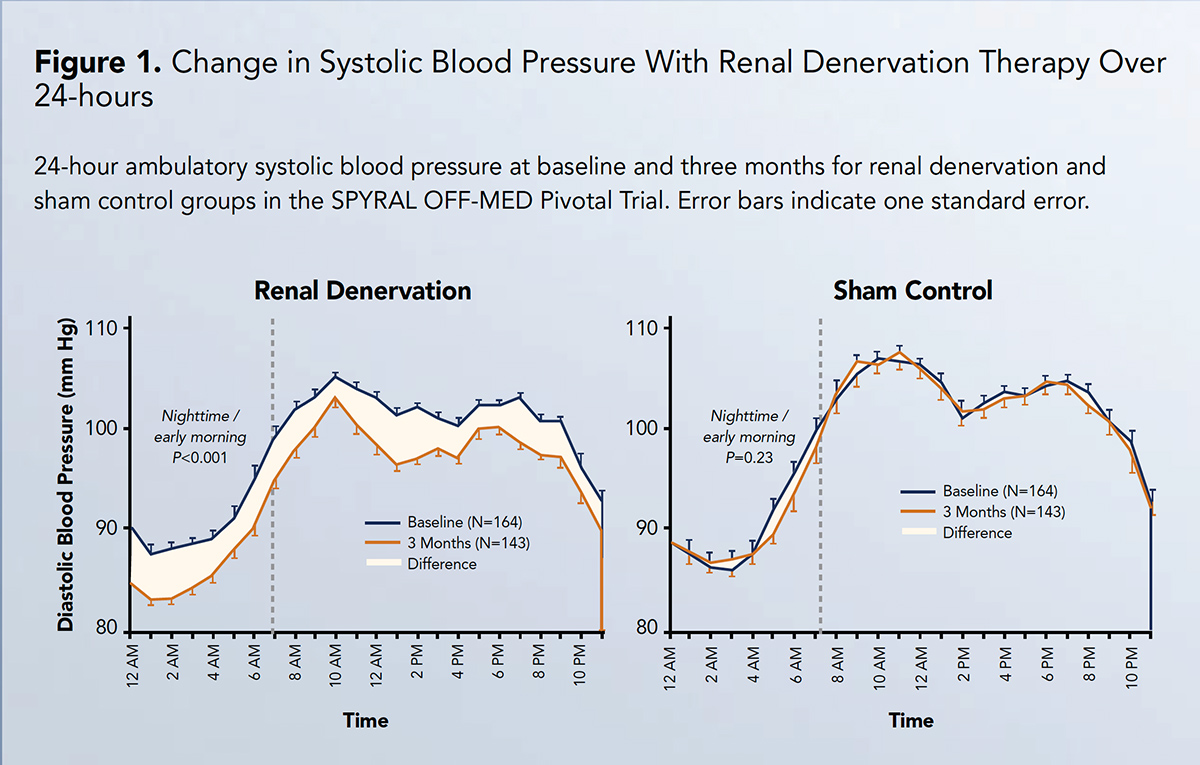
Reductions in blood pressure after RDN were consistent throughout 24 hours, with significant differences in nighttime and daytime blood pressure from baseline to three months among RDN patients (Figure 1).
Treatment differences for 24-hour systolic blood pressure among patients with prespecified baseline characteristics demonstrated no significant interactions between subgroups in SPYRAL HTN OFF-MED.
Moreover, there were no major device- or procedural-safety events through three months.
To the latter point, assurance of safety is an important finding given the advancement in RDN technique using a multi-electrode catheter that permitted simultaneous or sequential energy delivery to the main renal arteries with extension into distal renal artery branches to enable more complete, circumferential ablative treatment.
As the largest randomized evaluation of RDN in the absence of antihypertensive medications, the SPYRAL HTN OFF-MED trial confirms the effectiveness of RDN to reduce blood pressure and provides further reassurance related to safety specific to this technology and method.
In particular, RDN was associated with significant reductions in both systolic and diastolic blood pressure measured both over 24 hours and in the office setting. The persistent reduction ("always on" effect; Figure 1) in blood pressure achieved with RDN over a 24-hour period distinguishes this therapy from limitations associated with pharmacokinetic profiles and dosing regimens of medications, in addition to medical adherence.
Further, the consistent reduction in blood pressure at all timepoints may have special relevance for individuals whose blood pressure phenotype conveys high cardiovascular risk, for example, those with nocturnal or early morning hypertension.
The relevance of the blood pressure reductions observed in the SPYRAL HTN OFF-MED trial is an ongoing subject of interest. In prior studies of pharmaceutical therapies, a 10 mm Hg blood pressure decline in office systolic blood pressure – similar with that observed in this trial – is associated with an approximately 25% relative reductions in stroke and cardiac events, in addition to nearly a 15% reduction in mortality.8

Although the benefit of RDN in the present trial is similar with that achieved with a single antihypertensive drug, previous studies have demonstrated a positive association between magnitude of blood pressure reduction and baseline blood pressure severity.8
In addition, progressive lowering of blood pressure over longer timelines has been observed following RDN.4
Finally, the results of the SPYRAL HTN OFF-MED trial may also represent a conservative estimate of the blood pressure lowering effect; prior to the three-month primary endpoint, approximately twice as many patients in the sham control group compared with the RDN group resumed medications under protocol-defined "escape" criteria (p=0.049), either because of systolic blood pressures exceeding 180 mm Hg or due to blood pressure-related symptoms or complications.
Consequently, a greater number of patients with large increases in systolic blood pressure after randomization were underrepresented in the control group, because these subjects were usually unable to complete 24-hour ambulatory blood pressure monitoring for the primary endpoint assessment prior to resuming medications.
Altogether, this and other recent studies not only reaffirm the biologic proof of principle for this novel treatment but also offer insight to its complementary benefit in uncontrolled hypertension despite prescribed medications.
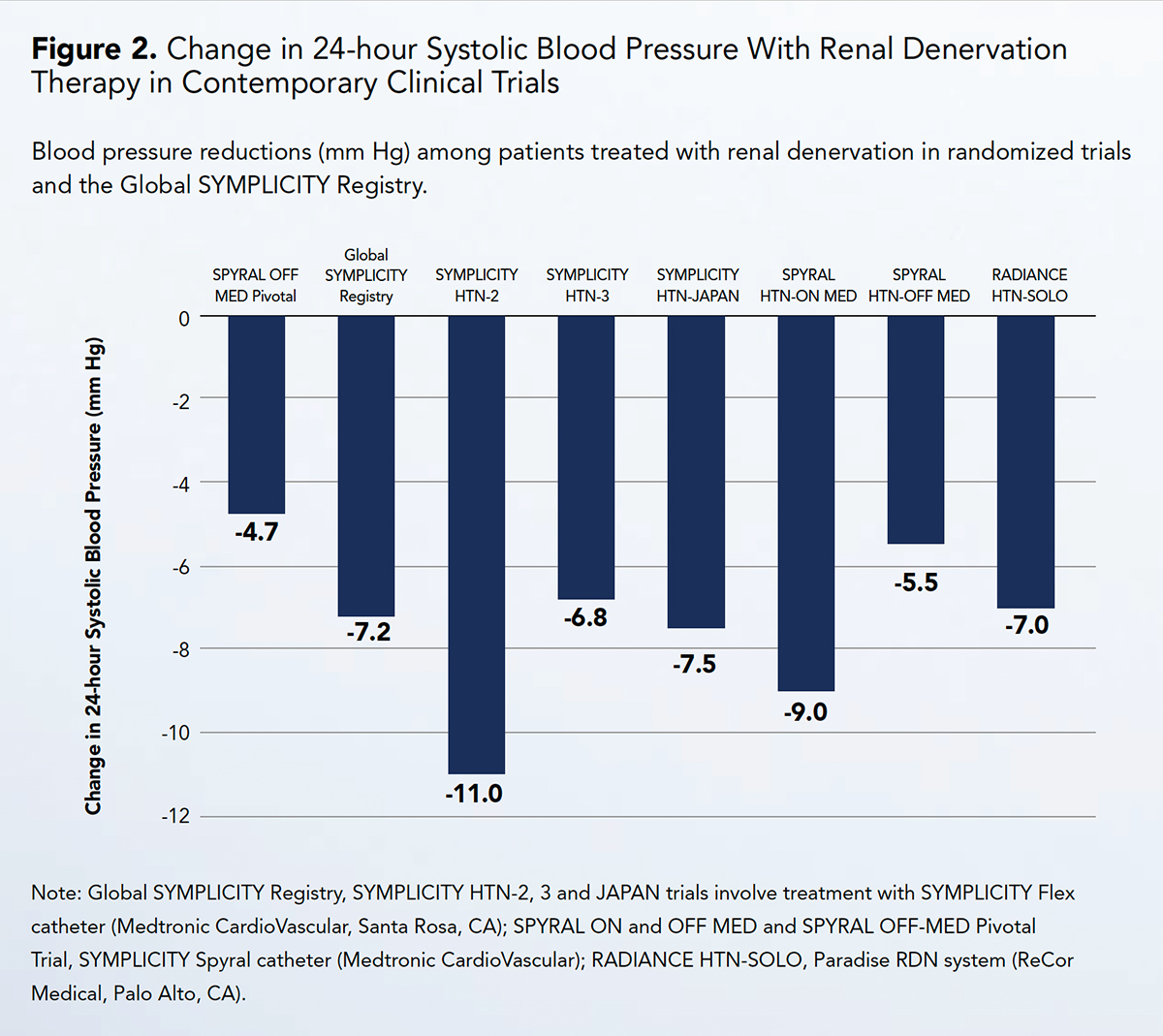
Across studies of varied design and even with different methods, consistent and clinically meaningful reductions in blood pressure have been achieved with RDN (Figure 2).
With this momentum, additional studies are intended to position RDN as part of standard therapy for the world's leading cause of death and disability.
Aside from the SPYRAL HTN OFF-MED trial, additional study is underway in patients with uncontrolled hypertension despite prescribed medical therapy, a setting more representative of clinical practice for which integrating drug and procedural strategy may be anticipated (NCT02439775).
In parallel, studies of similar design yet with other novel methods to accomplish RDN are ongoing (NCT03614260; NCT02910414).
Identifying predictors of treatment effect is an evolving area of focus to inform patient selection and expectations. Equally important, studies are underway to demonstrate durability of blood pressure lowering amidst unpredictable patient and prescriber behavior and coexisting health conditions that may influence blood pressure.
Finally, with awareness of a high prevalence of medication nonadherence in hypertension treatment, patient preference and tolerance of risk/benefit balance with both medications and device-based therapy is a focus of forthcoming study.
References
- Bhatt DL, Kandzari DE, O'Neill WW, et al., for the SYMPLICITY HTN-3 Investigators. N Engl J Med 2014;370:1393-1401.
- Kandzari DE, Bhatt DL, Brar S, et al. Eur Heart J 2015;36:219-27.
- Townsend RR, Mahfoud F, Kandzari DE, et al., for the SPYRAL HTN-OFF MED Trial Investigators. Lancet 2017;390:2160-70.
- Kandzari DE, Böhm M, Mahfoud F, et al., for the SPYRAL HTN-ON MED Trial Investigators. Lancet 2018;391:2346-55.
- Azizi M, Schmieder RE, Mahfoud F, et al., for the RADIANCE-HTN Investigators. Lancet 2018;391:2335-45.
- Böhm M, Townsend RR, Kario K, et al. Clin Res Cardiol 2020;109:289-302.
- Böhm M, Kario K, Kandzari DE, et al., for the SPYRAL HTN-OFF MED Pivotal Investigators. Lancet 2020;395:1444-51.
- Ettehad D, Emdin CA, Kiran A, et al. Lancet 2016;387:957-67.
Keywords: ACC Publications, Cardiology Magazine, Blood Pressure, Antihypertensive Agents, Control Groups, Renal Artery, Blood Pressure Monitoring, Ambulatory, Confounding Factors, Epidemiologic, Medication Adherence, Patient Selection, Patient Preference, Prevalence, Follow-Up Studies, Bayes Theorem, Pilot Projects, Cardiovascular Diseases, Cause of Death
< Back to Listings



