Cover Story | The Future of Digital Health in Cardiovascular Disease: Bridging Innovation and Clinical Practice

Cardiovascular diseases, including cardiometabolic diseases and related conditions, are the leading causes of death and disability worldwide.1 Despite advances in pharmacological treatments, significant gaps remain in achieving optimal cardiometabolic health, with disparities and barriers to accessing care.
Digital health technologies (DHTs), such as remote patient monitoring, virtual care and artificial intelligence (AI) data analysis, offer realistic opportunities to improve management strategies and preventive efforts. They are key to future primary prevention, secondary prevention and treatment paradigms in cardiovascular disease. However, DHT adoption in health care remains limited due to barriers in evidence generation linking DHTs to sustained improved clinical outcomes, challenges to clinical workflow integration and disparities in patient access to certain technologies. We explore strategies to overcome these barriers, ensuring that we can unlock the full potential of DHTs and revolutionize modern cardiovascular disease care.
Building an Evidence Base For DHTs
Widespread adoption of DHTs is limited by a lack of robust evidence linking their use to improved clinical outcomes. Evidence generation for clinical outcomes in cardiovascular disease are historically demonstrated in large trials, over the course of several years, and with high cost.2 The primary goals are to determine efficacy and effectiveness, understand potential for harm and quantify benefit. Developers, regulatory agencies, professional societies and end-users of DHTs will need to align on the balance between benefit and risk, and how to bring potentially beneficial DHTs to clinical use without long timeframes and without high cost.
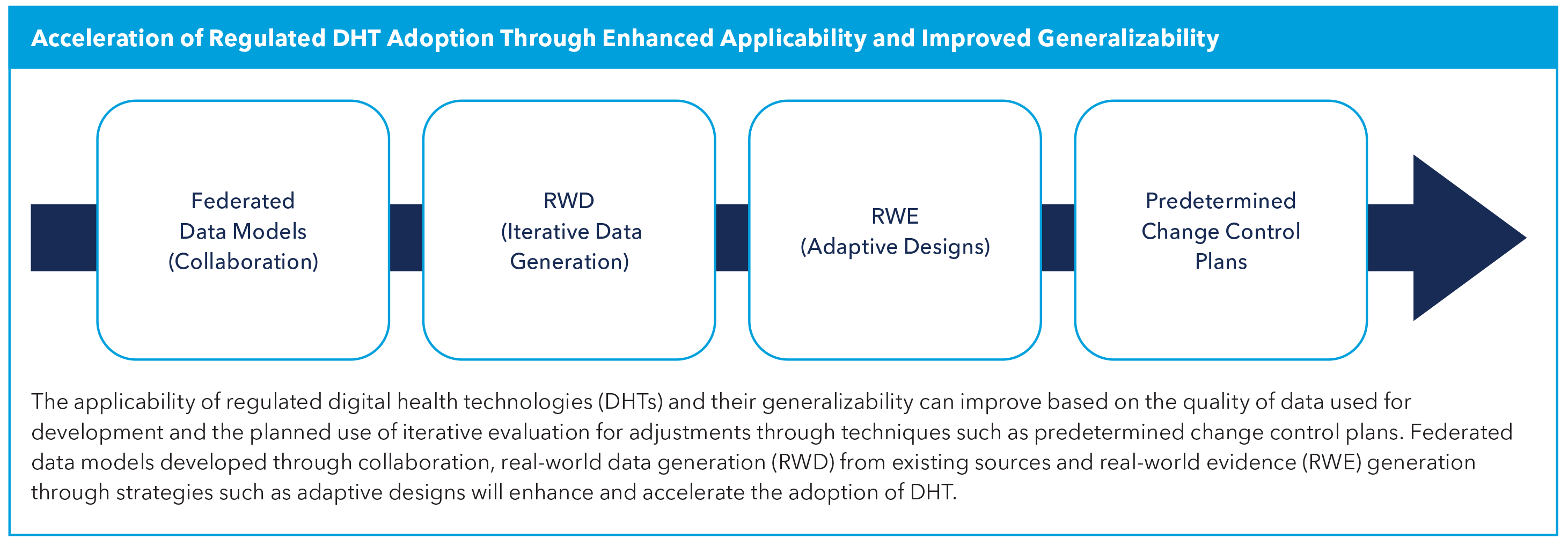
Most importantly, given the pace of change and rapid evolution of certain technological capabilities, including AI, the ecosystem's stakeholders will need to adopt adaptive and agile assessment techniques. Strategies might include the use of surrogate endpoints with clear guidance, acceptance of novel outcomes measures and real-world evidence (RWE).
Regulatory Innovation For AI in Cardiology
Since 1995, the U.S. Food and Drug Administration (FDA) has approved about 1,000 AI-enabled medical devices, with a sharp increase in recent years.3 All of the approvals employed rigorous criteria to assess safety and effectiveness. However, different from the evidence and testing required for marketed pharmacotherapies, some of the premarket approval validation and testing of DHTs may occur in a retrospective or ex-vivo manner. While that lends a certain efficiency to design, AI algorithms are dependent on the data used to develop them, but generalizability may be limited since they are not tested in a prospective manner in the applicable populations.

Limited data sets for training and validation can perpetuate bias, and these models are not applicable to more diverse populations. To improve the applicability of the algorithms, developers may consider federated learning approaches which have demonstrated better accuracy by using a collaborative approach to include data from diverse data sets, with utmost care to preserve data privacy and security.4 It is the responsibility of the developers of AI algorithms to consider sources of bias and mitigation strategies at each stage of design, development and implementation.5

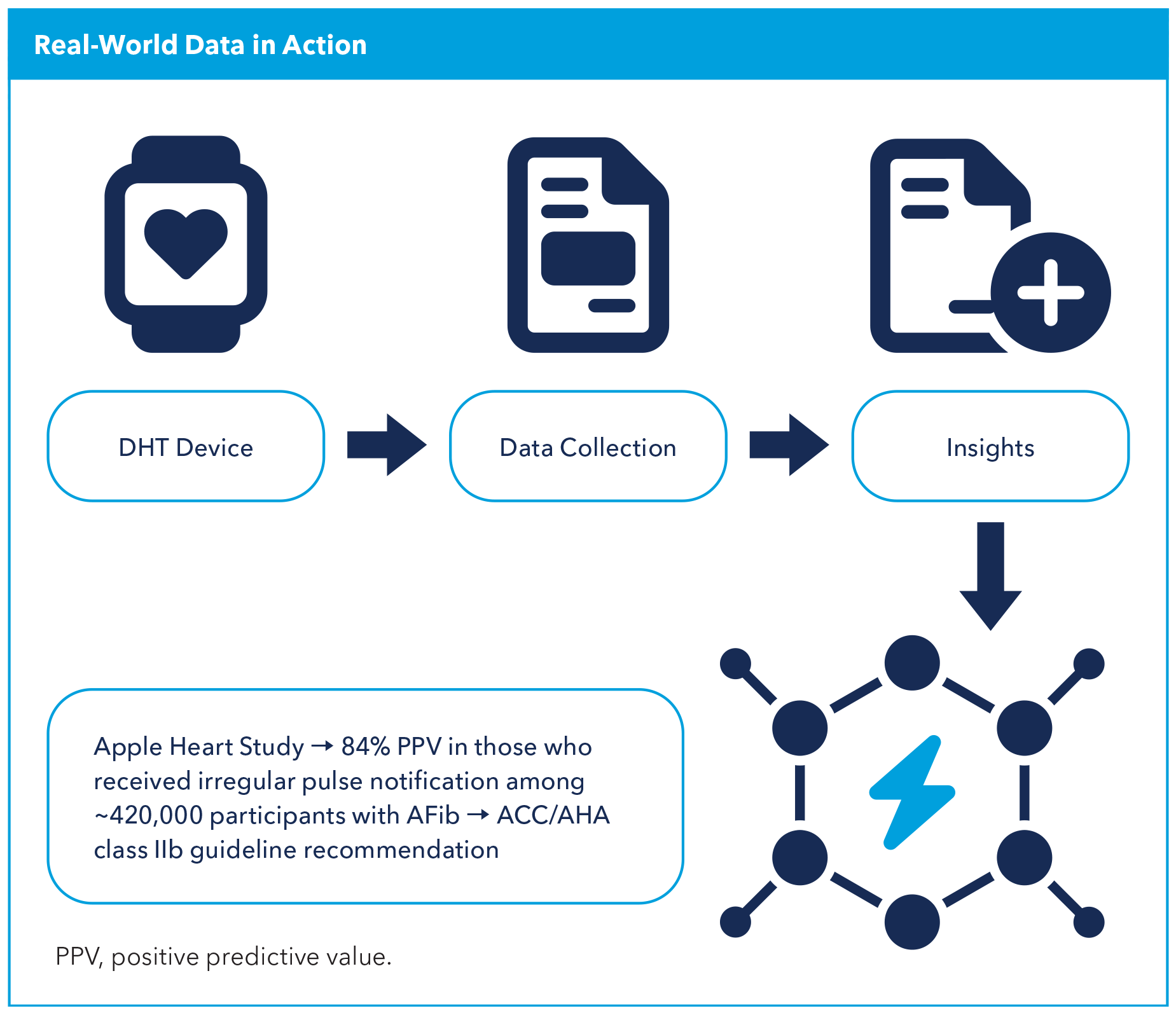
Once approved and marketed, DHT data should be efficiently collected to evaluate its real-world effectiveness. This evidence can then inform professional societies for guideline adoption, developers for agile algorithm modifications and end-users for continued utilization. For example, data from the Apple Heart Study showed that out of 419,297 enrolled patients there was a 34% incidence of atrial fibrillation (AFib) with an 84% positive predictive value among those who received an irregular pulse notification, demonstrating that wearable-based screening is feasible and has reasonable predictive accuracy.6 Subsequently, a class IIb recommendation was included in the ACC/ American Heart Association guideline on AFib stating that "wearable or handheld devices may be useful to identify undiagnosed [AFib] in select at-risk individuals."7
Real-world data (RWD) and RWE generation are already part of the fabric of routine clinical practice and postmarket surveillance, and they are increasingly used for premarket approval and indication expansion. It is imperative that novel approaches continue to be validated with generation of RWE, even in simple pragmatic studies. Use of these adaptive techniques will reduce timelines and generate robust data for the evaluation of risk and benefit of DHTs, as well as support iterative improvements and innovation.
The FDA has updated the guidance on the use of RWE for the regulatory decision-making process for medical devices,8 and when it is finalized it will replace the 2017 guidance. The FDA states that RWE may constitute valid scientific evidence and may be used to support regulatory decisions. Further, the FDA acknowledges that RWE may constitute an efficient means of generating data to inform regulatory decisions, especially in some cases where traditional clinical studies may be impractical to conduct.
In the guidance document, the FDA outlines reliable data sources such as registries, electronic medical records (EMRs), patient-generated data and device-generated data, and highlights circumstances where the use of this RWD is appropriate to generate RWE. This guidance and commitment to expanding the use of high-quality data to expedite the medical device regulatory process in safe and effective ways highlights the commitment made to keep pace with technological advancements and progress.
The FDA Center for Devices and Radiological Health has a predetermined change control plan (PCCP) which supports iterative improvement through prospective FDA review and authorization of modifications to an AI/machine learning (ML)-enabled device software function, while continuing to provide a reasonable assurance of device safety and effectiveness.
In other device-specific guidance, the FDA has also recommended using PCCPs to implement specific modifications for certain device types. This guidance offers a framework for similar post deployment monitoring and iteration plans and will likely be essential in creating efficient and safe DHT adoption.9
Despite regulatory review, it is imperative to review the effectiveness of DHT in clinical practice, and even more important to consider local evaluation and adoption strategies in the context of prevailing regulations. New care pathways may need to be created to support the implementation of these solutions. For example, the TIM-HF2 study published in The Lancet in 2018 showed clinically meaningful benefit when the use of remote care was tightly integrated with clinical teams.10
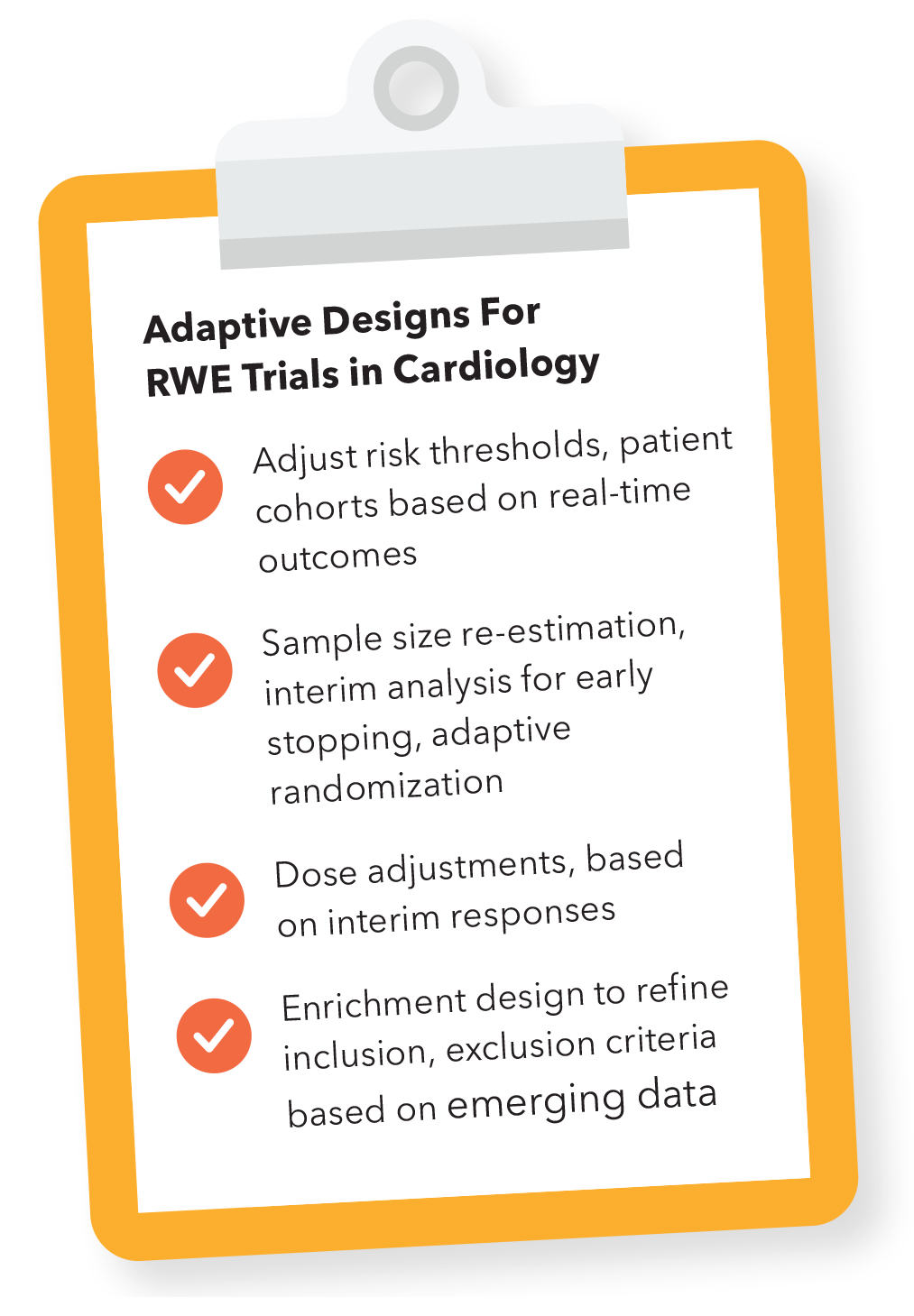
Regulated technologies may also consider the process required to expand their indication for use. DHT developers and researchers may look to RWE for potential study design to expand use. Adaptive designs in cardiology RWE trials have been crucial for dynamically optimizing AI models by adjusting risk thresholds and patient cohorts based on real-time outcomes, enabling continuous refinement that enhances the model's generalizability and clinical utility across diverse populations.
These adaptive features are essential in prospective clinical trials and during retrospective RWE collection in quality studies of clinical practice, as well as in post deployment iteration plans. These features include sample size re-estimation, interim analysis for early stopping and adaptive randomization. Sample size re-estimation allows adjustment of participant numbers based on the effect size observed during interim analysis, ensuring the trial remains powered to detect meaningful effects. Interim analysis can determine whether to stop a trial early for success or futility, while adaptive randomization dynamically allocates more participants to treatment arms showing better performance, improving both the efficiency and relevance of the trial.
Adaptive trials also include modifications like dose adjustments and enrichment designs. In dose modifications, treatment thresholds are fine-tuned based on interim responses to optimize outcomes, particularly when testing AI-guided interventions in cardiology. Enrichment designs refine inclusion or exclusion criteria based on emerging data, allowing the trial to focus on patient populations more likely to benefit, such as those at higher risk of cardiovascular events. The FDA has also issued guidance on the use of adaptive methods in clinical trials.11
Overcoming Clinical Integration Barriers
As cardiovascular care delivery systems create more efficient regulatory processes to assess the risk, benefit and safety of DHTs, it is equally important to consider the level of professional acceptance regarding the evidence supporting these innovations. Effective regulation not only requires streamlined assessments, but it also necessitates the backing of clinical practitioners and stakeholders who rely on credible evidence to integrate DHTs into practice. There needs to be a collaborative effort between developers and end-users to ensure data-driven insights that add value in an environment where clinicians are already burdened by the tremendous amount of data. Balancing regulatory efficiency with the rigor of evidence that ensures professional trust in these technologies is essential for their successful adoption and for advancing patient care.
DHTs face challenges in clinical integration, as many exist as standalone apps that do not easily fit into existing health care systems. Furthermore, there is often a failure to adopt immature user interfaces and poor user experience. This disconnection creates barriers for clinicians, patients and payers, impeding broader adoption. Health systems may consider internally built tools to optimize integration and adoption to address needs unique to a limited user base. However, if the needs are more pervasive, it may be useful to adopt an external tool – but face potential challenges in integration. Frequent assessments of performance, usability and end-user satisfaction are key to maintaining these solutions.12 Ideally, solutions with clinician-facing elements will fit seamlessly into clinical workflows through EMR integration, however this is often challenging to accomplish due to the lack of interoperability of EMRs.
Redefining Success: The Gold Standard For DHTs in Medicine
In setting the gold standard for DHT and AI in medicine, it is essential to measure success against current guideline adherence and real-world outcomes rather than limiting comparisons to clinical trial benchmarks in cardiometabolic diseases. A regulatory framework to create an infrastructure with guardrails for iterative improvement can ensure that novel tools are continuously refined, fostering forward progress that goes beyond initial success and drives ongoing enhancements in patient care, with results exceeding existing real-world practice. This approach promises a dynamic and impactful future for AI in medicine, where technology adapts to and meets the evolving needs of clinical practice.
To unlock the full potential of DHTs, there is a dependence on rigorous evidence generation, improved clinical integration and equitable access.13 Strengthening the evidence base for DHTs could lead to better payer coverage and broader adoption, particularly for populations most in need. Integration with the EMR, reduced trial costs and the ability to share data safely will result in streamlined clinical workflows, shared cost savings, and improvement in algorithm performance and inclusiveness. Collaboration across the health care ecosystem is essential to ensure DHTs contribute meaningfully to the prevention, diagnosis and treatment of cardiovascular diseases. By addressing these challenges, the health care ecosystem can unlock the full potential of digital health, transforming cardiovascular disease management and improving patient outcomes on a global scale.
This article was authored by Nivee Amin, MD, MHS, FACC, Division of Cardiology, Weill Cornell Medical College, New York, NY; Ashley N. Beecy, MD, FACC, Digital Health Team, Sutter Health, Sacramento, CA; and Ami B. Bhatt, MD, FACC, chief innovation officer of the ACC.
References
- Vaduganathan M, Mensah G, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol 2022; 80):2361-71.
- Oikonomou EK, Thangaraj PM, Bhatt DL, et al. An explainable machine learning-based phenomapping strategy for adaptive predictive enrichment in randomized clinical trials. NPJ Digit Med 2023; 6: 217.
- Warraich HJ, Tazbaz T, Califf RM. FDA perspective on the regulation of artificial intelligence in health care and biomedicine. JAMA 2025;333:241-7.
- Goto S, Solanki D, John JE, et al. Multinational federated learning approach to train ECG and echocardiographic models for hypertrophic cardiomyopathy detection. Circulation 2022;146:755-69.
- Abràmoff MD, Tarver ME, Loyo-Berrios N, et al. Considerations for addressing bias in artificial intelligence for health equity. NPJ Digit Med 2023; 6:170.
- Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909-17.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation. J Am Coll Cardiol 2024;83:109-279.
- Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices. Draft guidance for Industry and FDA staff. December 2023. Accessed Nov. 15, 2024. Available here.
- Predetermined Change Control Plans for Medical Devices: Draft Guidance for Industry and Food and Drug Administration Staff, FDA, Center for Devices and Radiological Health. August 2024. Accessed Nov. 15, 2024. Available here.
- Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 2018;392:1047-57.
- Adaptive Designs for Medical Device Clinical Studies Guidance for Industry and Food and Drug Administration Staff, FDA, Center for Devices and Radiological Health. July 2016. Accessed Nov. 15, 2024. Available here.
- Marwaha JS, Landman AB, Brat GA, et al. Deploying digital health tools within large, complex health systems: key considerations for adoption and implementation. NPJ Digit Med 2022; 5:13.
- Warraich HJ, Patrick-Lake B, Saha A, et al. Digital health technologies for cardiometabolic disease and diabetes: a perspective from the U.S. Food and Drug Administration. J Am Coll Cardiol 2025;85:528-35.
Keywords: Cardiology Magazine, ACC Publications, Cardiovascular Diseases, Health, Digital Technology, Artificial Intelligence, Wearable Electronic Devices, Innovation


