Feature | The Overlooked Cardiac Implications of Alpha-Gal Syndrome
Life-altering consequences of a tick-bite

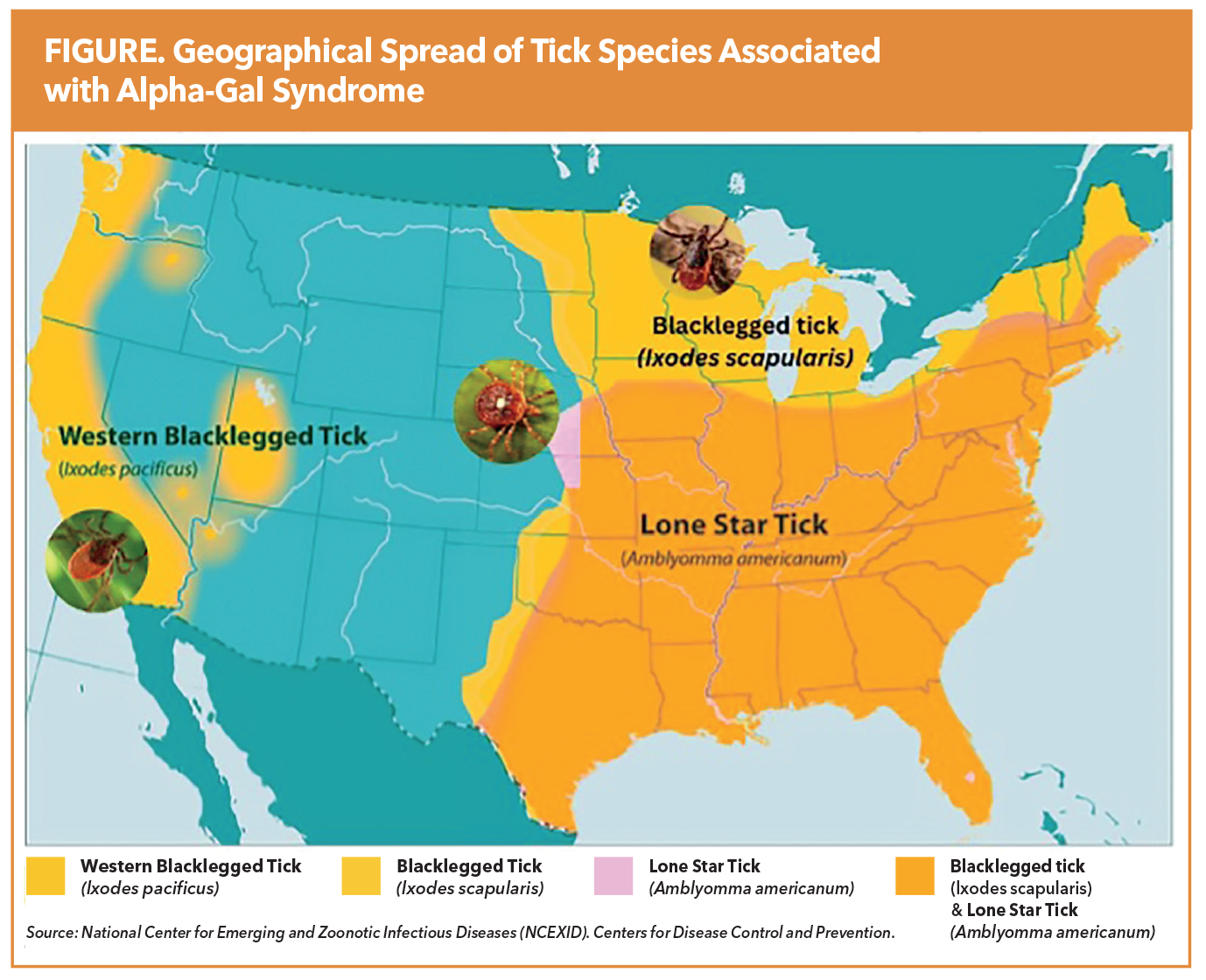
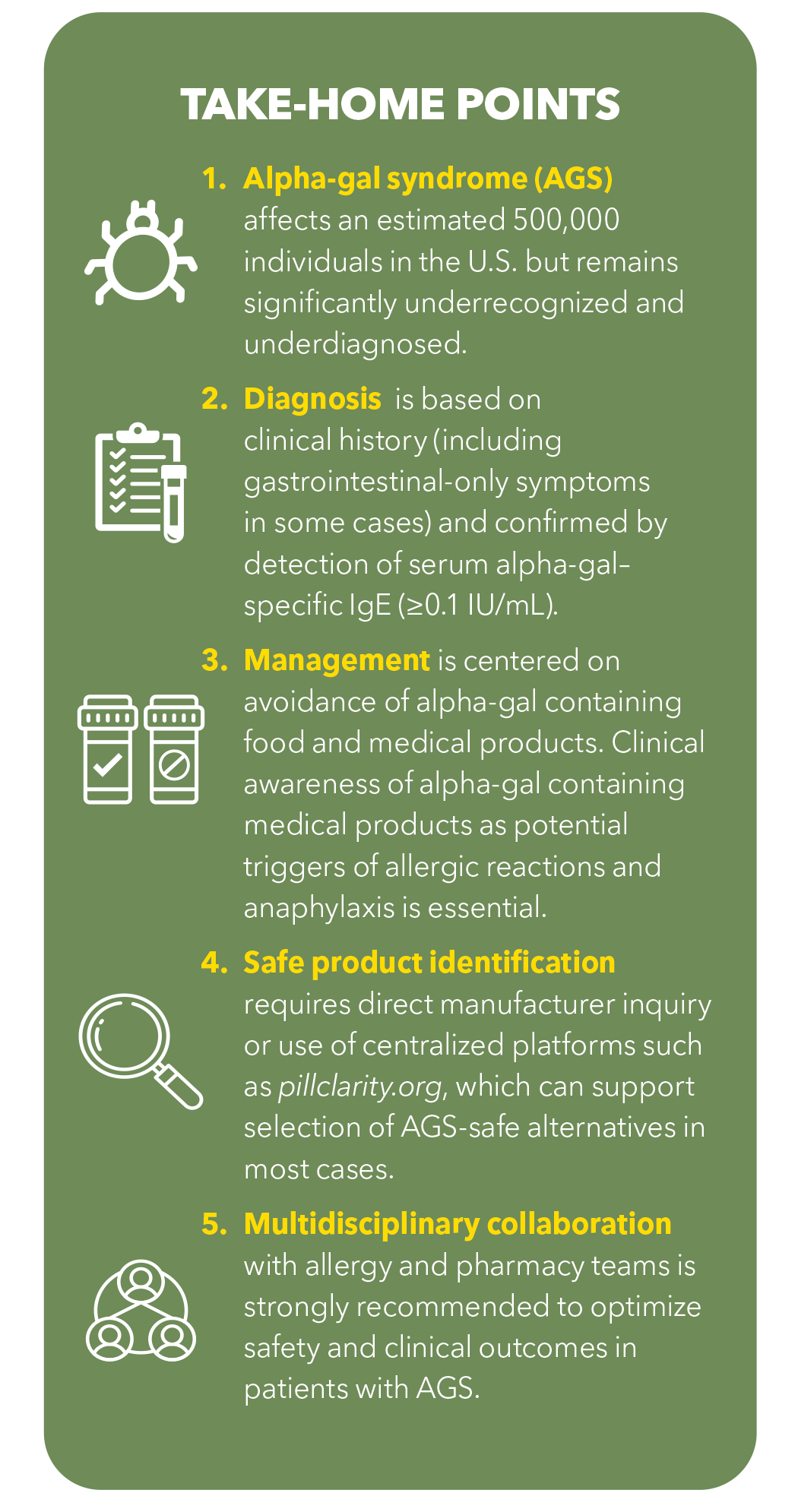
Alpha-Gal Syndrome (AGS) is an underrecognized, tick-borne allergic condition with emerging relevance in cardiovascular medicine. Sensitization to galactose-α-1,3-galactose (alpha-gal), a mammalian-derived carbohydrate absent in humans and other primates, occurs following tick bites, most commonly from the Lone Star tick (Amblyomma americanum). More recently, the blacklegged tick (Ixodes scapularis) and the western blacklegged tick (Ixodes pacificus) have also been implicated in alpha-gal sensitization.
The condition is most prevalent in the contiguous region spanning the southern, midwestern and mid-Atlantic U.S., particularly parts of Oklahoma, Kansas, Arkansas, Missouri, Mississippi, Tennessee, Kentucky, Illinois, Indiana, North Carolina, Virginia, Maryland, and Delaware (Figure). According to the Centers for Disease Control and Prevention, 450,000 Americans may be affected by AGS, with sensitization rates exceeding 35% in certain endemic areas. Despite this substantial disease burden, AGS remains significantly underdiagnosed, and many clinicians are unfamiliar with its clinical presentation or implications.
Clinically, AGS is characterized by a delayed IgE-mediated hypersensitivity reaction, typically occurring two to six hours after ingestion or parenteral exposure to alpha-gal–containing products. Clinical manifestations range from isolated gastrointestinal symptoms and/or urticaria to life-threatening anaphylaxis. Notably, up to 10% of patients diagnosed with idiopathic anaphylaxis in endemic regions have subsequently been found to have AGS. Diagnosis is based on a suggestive clinical history in combination with detectable serum alpha-gal–specific IgE (≥0.1 IU/mL).
Emerging evidence suggests that AGS, traditionally associated with red meat allergy, may have important pathophysiologic links to coronary artery disease (CAD). In a prospective study of 118 patients undergoing coronary catheterization with IVUS, the presence of alpha-gal–specific IgE was associated with significantly greater atheroma burden and high-risk plaque morphology, including increased calcification, fibrofatty content and necrotic core volume.1 These features are hallmarks of vulnerable plaque, known to predict adverse cardiovascular outcomes. The association was especially pronounced in patients aged ≤65 years, raising concern for accelerated atherogenesis in sensitized individuals. These findings were further supported by data from the Australian BioHEART cohort (n=1,056), where α-Gal sensitization was independently associated with noncalcified plaque burden and obstructive CAD and occurred at higher rates in STEMI patients than those with stable or no CAD.2 Together, these studies suggest a possible role for chronic immune activation or dietary alpha-gal exposure in promoting vascular inflammation and plaque instability.
Beyond mechanistic concerns, AGS has practical implications for cardiovascular care. Many commonly used medications and devices contain mammalian-derived components, which may trigger allergic reactions in sensitized individuals. This highlights the importance of recognizing AGS not only as a systemic allergic condition but also as a potential cardiovascular risk modifier that intersects with both pharmacologic safety and atherosclerotic disease biology.
Medication and Device Related Risks in CV Care
A significant concern in cardiology is the widespread use of medications and devices containing mammalian-derived ingredients (Table). A review found that 74% of the 100 most commonly prescribed medications contain components such as gelatin, magnesium stearate or lactose, which may carry alpha-gal or contain residual alpha-gal.3 According to a 2023 survey of 559 AGS patients, 92% had to change medications due to reactions, 75% reported adverse effects and 50% experienced anaphylaxis.4
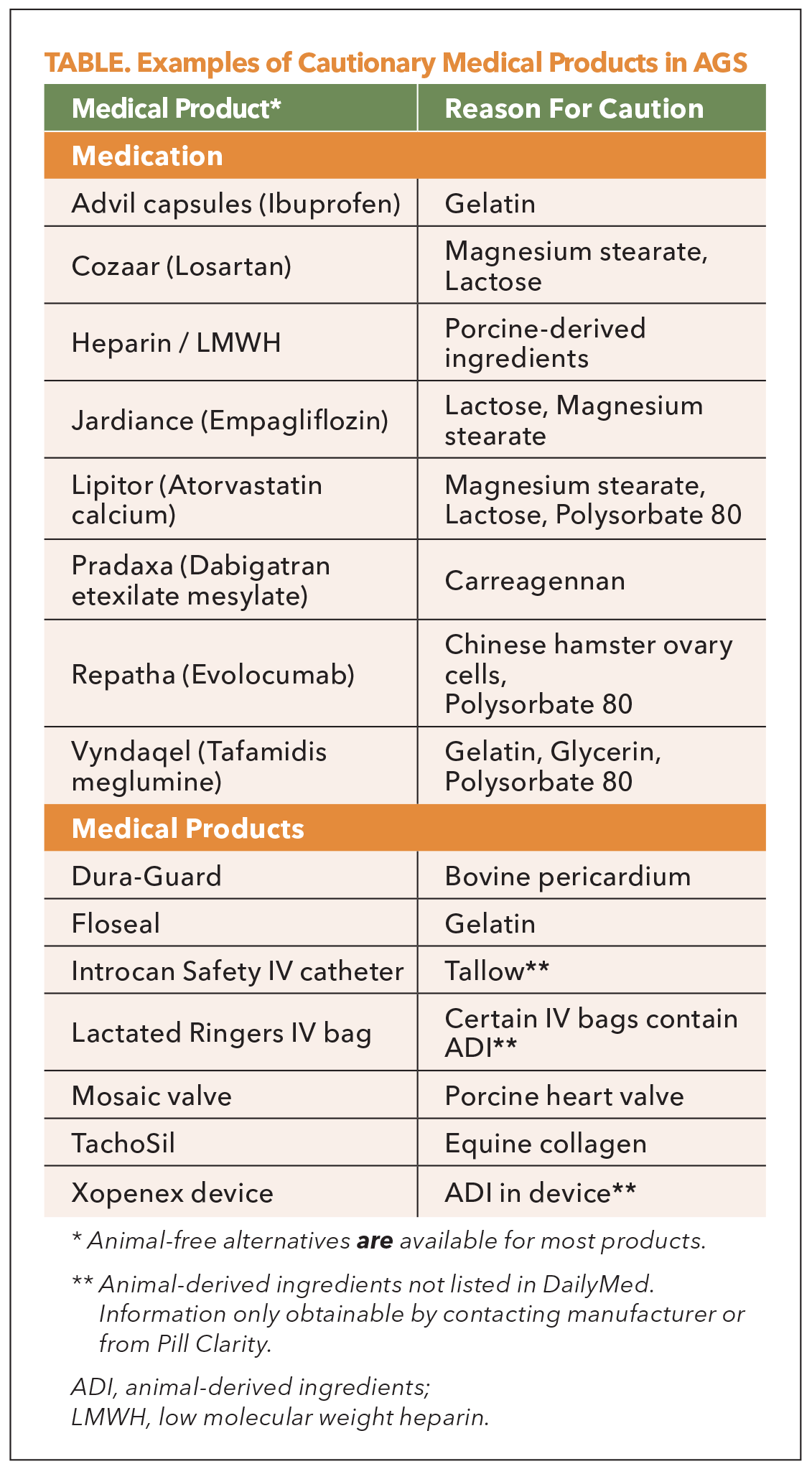
Unfractionated heparin, derived from porcine intestine or bovine lung, remains the standard anticoagulant in many cardiac and vascular procedures. However, it has been implicated in severe allergic reactions in AGS patients, with one study reporting up to a 50% reaction rate during cardiopulmonary bypass.5 While alternatives such as bivalirudin or argatroban exist, their use requires case-by-case evaluation. Alpha-gal IgE screening and pretreatment with corticosteroids may be prudent for elective surgeries.
Low-molecular-weight heparins, such as enoxaparin, also pose risks due to their porcine origin. Gelatin-containing hemostatic agents (e.g., Gelfoam, Surgifoam), commonly employed in cardiovascular procedures, and certain monoclonal antibodies (e.g., cetuximab) present additional concerns. Notably, a cluster of unexpected and severe infusion reactions, including fatal anaphylaxis associated with cetuximab administration in the southeastern U.S., was pivotal in identifying the underlying role of alpha-gal sensitization and led to the initial recognition of AGS. Furthermore, bioprosthetic valves and vascular grafts derived from bovine or porcine tissues contain alpha-gal epitopes, which may trigger immediate hypersensitivity reactions or contribute to chronic immunologic responses and premature structural degeneration.
Blood transfusion practices may also need adjustment. Due to molecular mimicry between the B blood group antigen and alpha-gal, individuals with blood type A or O may be more prone to cross-reactivity. Avoidance of type B red blood cell products in these patients could reduce the risk of transfusion-related reactions.
Even medications typically perceived as low risk, such as atorvastatin, may warrant closer scrutiny. With more than 40 suppliers in the U.S. market, only some are absent of any mammalian-derived ingredients and provide transparent documentation. Conversely, dabigatran (Pradaxa), although free from animal-derived ingredients, can contain carrageenan a seaweed-derived polysaccharide that structurally mimics the alpha-gal epitope. Importantly, this additive is present in the brand-name formulation but is absent in select generic versions. Such examples underscore the need for excipient-level evaluation, vigilance of supplier-based formulation variability, implications on outpatient and hospital-supplied versions, and the multiple pathways of unintentional alpha-gal exposure.
No consensus guidelines exist for perioperative screening and management of AGS in cardiology, but expert recommendations suggest that cardiologists consult with allergy and immunology specialists when managing sensitized patients. Preoperative screening for alpha-gal IgE, careful review of all drugs and devices to be used, and consideration of alternatives when high-risk components are identified are all advised. This is particularly critical in high-stakes interventions such as catheterization, valve replacement or bypass surgery.
Navigating Safe Product Selection: Resources and Systems-Based Solutions
Given the variability in excipient formulations, accurately identifying alpha-gal–safe products is complex. Resources like DailyMed (dailymed.gov) and manufacturer call centers can offer some solutions, but limitations include inconsistent response quality, and at times discordant excipient listings. Clinicians are left to perform labor-intensive due diligence, which is cumbersome when accounting for multiple medications from a variety of manufacturers.
Pill Clarity (pillclarity.org), a pharmacist-run national medical information platform, serves as a centralized solution for identifying medications and over-the-counter products free from mammalian-derived ingredients. This resource supports clinicians and patients alike, addressing a major gap in allergen transparency. Pill Clarity also serves other vulnerable populations, including the 33 million Americans with food allergies, those with dietary preferences (e.g., vegan) and individuals requiring religious dietary accommodations (e.g., Kosher, Halal). Based on their data presented at the 2025 American Academy of Allergy Asthma and Immunology/World Allergy Organization conference, an AGS-safe alternative can be identified in over 75% of cases.
Since drug manufacturers may modify suppliers or reformulate products over time, there is a need for ongoing vigilance and dynamic information systems. Manufacturers are encouraged to keep databases such as DailyMed up to date and to collaborate with specialized platforms like Pill Clarity that focus on excipient transparency. One such example is Furoscix (furosemide injection), delivered via an on-body infuser system. Its complexity comprising active and inactive drug components, a container and delivery device creates multiple points of potential exposure to animal-derived ingredients. In response, its manufacturer has proactively disclosed information on over 50 common allergens, offering accessible documentation to patients and providers upon request.
Momentum for regulatory reform is also increasing. In 2018, the American Medical Association adopted a policy supporting mandatory disclosure of animal-derived ingredients in pharmaceuticals. Building on this, a 2023 Citizen Petition to the U.S. Food and Drug Administration called for standardized and transparent labeling of animal-derived components in medications and devices. The American Pharmacists Association has since endorsed these efforts, citing critical patient safety concerns and the practical challenges faced by clinicians.
In parallel, bioengineering innovations such as alpha-gal–knockout pig tissues are being explored for future use in bioprosthetic devices. These engineered tissues could offer long-term, AGS-safe options for patients undergoing surgical interventions; however, they are still in early development and not yet widely available.
Until comprehensive regulatory and manufacturing solutions are in place, cardiologists must adopt a proactive approach. This includes partnering with pharmacists, verifying ingredients on a case-by-case basis, and working closely with allergy and immunology specialists. With AGS prevalence rising and its cardiovascular implications increasingly evident, the integration of educational, clinical and systems-level tools is essential to ensure safe and effective care for this vulnerable patient population.

This article was authored by Sachin A. Shah, PharmD, FACC, professor of Pharmacy at the Thomas J. Long School of Pharmacy, University of the Pacific, and co-founder of Pill Clarity, a free national medical information center.
References
- Wilson JM, Nguyen AT, Schuyler AJ, et al. IgE to the Mammalian Oligosaccharide Galactose-α-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics-Brief Report. Arterioscler Thromb Vasc Biol 2018;38:1665-9.
- Vernon ST, Kott KA, Hansen T, et al. Immunoglobulin E Sensitization to Mammalian Oligosaccharide Galactose-α-1,3 (α-Gal) Is Associated With Noncalcified Plaque, Obstructive Coronary Artery Disease, and ST-Segment-Elevated Myocardial Infarction. Arterioscler Thromb Vasc Biol 2022;42:352-61.
- Tieu L, Uchi J, Patel N, et al. Embracing Medication Needs of Patients based on Ethical, Dietary, and Religious Preferences. Am J Lifestyle Med 2022;18:351-63.
- Uchi J, et al. Understanding health related challenges in patients with alpha-gal syndrome. Presented at: DIA Annual Global Meeting; June 25-29, 2023; Boston. Available here.
- Hawkins RB, Wilson JM, Mehaffey JH, et al. Safety of Intravenous Heparin for Cardiac Surgery in Patients With Alpha-Gal Syndrome. Ann Thorac Surg 2021;111:1991-7.
Keywords: Cardiology Magazine, ACC Publications, Galactose, Anaphylaxis, Red Meat, Allergy and Immunology, Tick-Borne Diseases
